Annual Report 2018
Division of Developmental Therapeutics
Yasuhiro Matsumura, Masahiro Yasunaga, Yoshikatsu Koga, Hiroki Takashima, Hikaru Iwafuji, Ryo Tsumura , Hirobumi Fuchigami, Takahiro Anzai, Shigehiro Koganemaru, Takuya Shiraishi, Kaori Hayashi, Kenji Takashima, Yohei Tsuruta, Takaki Noguchi, Yoko Omichi, Makoto Wakatsuki, Madoka Nakayama, Mamiko Shimada, Mayumi Yamauchi, Shinji Saijo, Shingo Hanaoka
Introduction
The Division of Developmental Therapeutics has been involved in basic research on drug delivery systems (DDS) and antibody therapeutics including monoclonal antibody (mAb) development, antibody drug conjugate (ADC), and the mAb conjugated micelle system. We have applied patents of all mAbs established in our division (Table 1). We also investigate mechanisms of cancer-induced blood coagulation and are developing a new cancer diagnosis based on the cancer specific mAb. In addition to the research work, we are operating the Japan Clinical Oncology Group (JCOG) Tumor Repository.
Table 1. Patent application of monoclonal antibodies developed in our division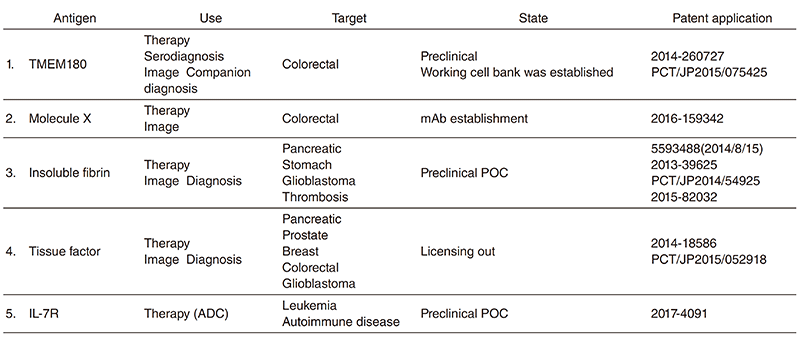

The Team and What We Do
- Development of antibody drugs and their derivation to pharmaceutical companies
- Examination of clinical trials as an IRB member
- Operation of the JCOG Tumor Repository
Research activities
1. Infrastructure for the mAb development
We have established an infrastructure for antibody development including antigen production, animalimmunization, hybridoma production, antibody expansion and purification, SPR characterization, and the enzymelinked immunosorbent assay development. Simultaneously, we have found various cell surface molecules specific to colorectal cancer and created mAbs that correspond to those molecules.
2. Cancer Stromal Targeting Therapy
In spite of recent success of ADC therapy in patients with hypervascular and special tumors recognized by a particular mAb, there are several issues to be solved for ADC to be counted as universal therapy for any types of cancer. Especially, most human solid tumors possess abundant stroma that hinders the distribution of ADC. To overcome these drawbacks, we developed a unique strategy that the cancerstromal targeting (CAST) therapy by cytotoxic immunoconjugate bound to the collagen 4, tissue factor (TF), or fibrin network in the tumor stroma from which the payload released gradually and distributed throughout the tumor, resulting in the arrest of tumor growth due to induced damage to tumor cells and tumor vessels.
We successfully developed a mAb (102-10 clone) that reacted only with human fibrin, not with human fibrinogen and cross-reacted with mouse fibrin but not with mouse fibrinogen. The specificity of our 102-10 differs from existing antifibrin mAbs. Namely, 102-10 reacts only with a fibrin clot, but not with fibrinogen, soluble fibrin, or D-dimer. The anti-fibrin antibody therefore did not make an immune complex in the bloodstream and circulated in the blood for a long time. We then prepared the ADC that is MMAE conjugated anti-fibrin mAb. The ADC may selectively extravasate from leaky tumor vessels, bind to the fibrin network in the stroma and create a scaffold from which effective sustained release of the free MMAE occurs. This free MMAE may easily reach cancer cells by diffusing through the stroma barrier. Another benefit is that MMAE released from the ADC may also attack the vascular and endothelial cells.
3. DDS in Cancer Chemotherapy
Tumor-targeted delivery of therapeutic agents is a longstanding pharmacological goal to improve the treatment selectivity and the therapeutic index. Most scientists have sought to use "active" receptor-mediated tumor-targeting systems. However, the "passive" targeting afforded by the "Enhanced Permeability and Retention (EPR) effect" provides a versatile and non-saturable approach for tumor-selective delivery. Polymeric micelles are ideally suited to exploit the EPR effect and have been used for the delivery of a range of anticancer drugs in preclinical and clinical studies. Anti-TF mAb conjugated micelle incorporating epirubicin exerted a potent antitumor effect in a human pancreatic cancer model, especially TF-highexpressing tumors.
4. Noninvasive Diagnostic Test for Colorectal Cancer
Regarding colorectal cancer (CRC), we investigated the applicability of the fecal miRNA test (FmiRT) to fecal samples used for a previous fecal occult blood test (FOBT) stored under various conditions.
Education
1) Doctoral students
Graduate School of Frontier Sciences, the University of Tokyo: two students Keio University School of Medicine: one student Nihon University School of Medicine: one student
2) Master course student
Graduate School of Frontier Sciences, the University of Tokyo: four students
Future prospects
The project of our team will be a hybrid of biology and physiology as this topic relates to tumors or tumor stromal biology as well as organic chemistry, and has the potential to establish a new field of drug design and to produce many potentially useful treatment modalities, especially for stroma-rich and refractory cancers such as pancreatic cancer, stomach cancer, CRC, and glioblastoma.
List of papers published in 2018
Journal
1. Yamazaki S, Higuchi Y, Ishibashi M, Hashimoto H, Yasunaga M, Matsumura Y, Tsuchihara K, Tsuboi M, Goto K, Ochiai A, Ishii G. Collagen type I induces EGFR-TKI resistance in EGFR-mutated cancer cells by mTOR activation through Akt-independent pathway. Cancer Sci, 109:2063-2073, 2018
2. Aung W, Tsuji AB, Sugyo A, Takashima H, Yasunaga M, Matsumura Y, Higashi T. Near-infrared photoimmunotherapy of pancreatic cancer using an indocyanine green-labeled anti-tissue factor antibody. World J Gastroenterol, 24:5491-5504, 2018
3. Hamaguchi T, Tsuji A, Yamaguchi K, Takeda K, Uetake H, Esaki T, Amagai K, Sakai D, Baba H, Kimura M, Matsumura Y, Tsukamoto T. A phase II study of NK012, a polymeric micelle formulation of SN-38, in unresectable, metastatic or recurrent colorectal cancer patients. Cancer Chemother Pharmacol, 82:1021-1029, 2018
4. Min HS, Kim HJ, Ahn J, Naito M, Hayashi K, Toh K, Kim BS, Matsumura Y, Kwon IC, Miyata K, Kataoka K. Tuned Density of Anti-Tissue Factor Antibody Fragment onto siRNA-Loaded Polyion Complex Micelles for Optimizing Targetability into Pancreatic Cancer Cells. Biomacromolecules, 19:2320-2329, 2018
5. Fuchigami H, Manabe S, Yasunaga M, Matsumura Y. Chemotherapy payload of anti-insoluble fibrin antibody-drug conjugate is released specifically upon binding to fibrin. Sci Rep, 8:14211, 2018
6. Prince R, Bologna L, Manetti M, Melchiorre D, Rosa I, Dewarrat N, Suardi S, Amini P, Fernandez JA, Burnier L, Quarroz C, Reina Caro MD, Matsumura Y, Kremer Hovinga JA, Griffin JH, Simon HU, Ibba-Manneschi L, Saller F, Calzavarini S, Angelillo-Scherrer A. Targeting anticoagulant protein S to improve hemostasis in hemophilia. Blood, 131:1360-1371, 2018
7. Tsumura R, Manabe S, Takashima H, Koga Y, Yasunaga M, Matsumura Y. Influence of the dissociation rate constant on the intra-tumor distribution of antibody-drug conjugate against tissue factor. J Control Release, 284:49-56, 2018
