Annual Report 2018
Department of Pharmacy
Masakazu Yamaguchi
Introduction
The Department of Pharmacy stores and dispenses drugs, prepares injections (including aseptic mixtures), collects and disseminates drug information, and provides patients with guidance regarding the proper use of drugs. Its services have been improved toward the hospital's goal of envisaging the highest quality of medical care, practice, and research. A state-of-the-art computerized system and other pharmacy-related equipment ensure quality control and inventory management, promote the proper use of drugs, and enhance the efficiency and quality of our services.
The Team and What We Do
As part of the fundamental function of the hospital, the Department of Pharmacy prepares and dispenses oral and topical medicines and injections for individual patients. All outpatients and inpatients are provided with aseptic mixtures of injectable chemotherapy agents prepared in our department. As the importance of providing drug information for patients has been widely acknowledged, clinical pharmacists visit inpatients and give advice on taking medicine, focusing especially on pain control with opioids, and participate in the palliative-care support team, while our department provides outpatients with guidance on the proper use of opioids and anti-cancer agents. Our department also places pharmacists in every hospital ward to provide medication reconciliation services for inpatients, with a view to enhance the quality of chemotherapy as well as to ease burdens on doctors and nurses. Pharmacists collect, compile, and maintain a database of drug information and distribute pertinent information to the medical staff. Drug information is disseminated quickly throughout the hospital by paper distribution and/or on the in-hospital computer network. Pharmacists individualize dosage regimens for specified drugs such as tacrolimus, aminoglycosides, and vancomycin based on both measured blood concentrations and pharmacokinetic analysis to maximize their efficacy, and to minimize adverse events. A physician places an order through the hospital's computerized electric medical record system. The prescription order is then redirected to the medicine-package-printing system which provides drug information. The medicine-package information, instructions and explanations, which are easy to understand for patients, for the proper use of drugs, such as those regarding efficacy and effectiveness, precautions, and guidance concerning symptoms in the early stage of adverse reactions, are automatically printed out for patients when a prescription is ordered. The injection-order is directly linked to an automatic "picking system" device, and this linkage ensures that injections are made properly and efficiently. This injectionordering system contains an additional function which is a regimen-ordering system for anticancer drugs that makes it possible to check the dose as well as the interval of chemotherapy. The Department of Pharmacy also has a robot which prepares injection preparations without human assistance.

Table 2. Amounts of Drugs Consumed in 2018
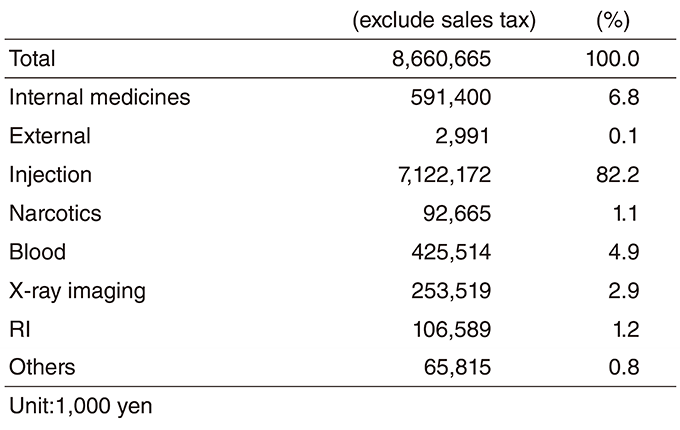

Table 3. Aseptic Preparation of Injectable Drugs in 2018


Table 4. Investigational Drugs


Research activities
Since an important mission of the Department of Pharmacy is to contribute to the development of new drugs, inventory control and handling of new investigative drugs are performed in accordance with Good Clinical Practice regulations. Research on the safety management of chemotherapy is conducted including handling of chemotherapeutic drugs, reduction of incidents regarding drugs, and improvement of pain control for patients who need palliative care through the use of guidance materials. A couple of studies on the pharmacokinetics and pharmacodynamics of cancer-related drugs have been performed and some of the results have been reported in international conferences and journals.
Education
The National Cancer Center Hospital offers a three-year postgraduate pharmacy residency training in clinical oncology. In the first year, the program places the most importance on the technical aspects of cancer care. In the second year, through required rotations in a variety of focused hematology/oncology services, the resident will refine his/her clinical problemsolving skills in cancer management and patient education, as well as provide pharmaceutical care to ambulatory care patients and participate in an oncology-focused Drug Information Program. In the third year, residents participate in specialized pharmaco-clinical practice and research activities, which may be tailored to the resident's goals. The hospital also provides a two-year chief residency program in which post-residency trainees may develop their clinical research capabilities to a higher level. Moreover, there are opportunities for educational activities, such as a training course for visiting expert pharmacists and postgraduate students of pharmacy, and participation in a multi-institutional TV conference.
Future prospects
In order to provide the best cancer care, the Department of Pharmacy promotes "the best cancer treatments", "developing and spreading of new treatments", "educating young pharmacists for oncology", and providing information to domestic and overseas organisations.
List of papers published in 2018
Journal
1. Tsuji D, Suzuki K, Kawasaki Y, Goto K, Matsui R, Seki N, Hashimoto H, Hama T, Yamanaka T, Yamamoto N, Itoh K. Risk factors associated with chemotherapy-induced nausea and vomiting in the triplet antiemetic regimen including palonosetron or granisetron for cisplatin-based chemotherapy: analysis of a randomized, double-blind controlled trial. Support Care Cancer, 27:1139-1147, 2019
2. Saito Y, Kumamoto T, Arima T, Shirakawa N, Ishimaru S, Sonoda T, Nakajima M, Sugiyama M, Arakawa A, Hashimoto H, Makino Y, Ogawa C, Yamaguchi M. Evaluation of aprepitant and fosaprepitant in pediatric patients. Pediatr Int, 61:235-239, 2019
3. Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, Nakao M, Sakai H, Nakayama T, Minato K, Arai T, Suzuki K, Shimada Y, Nagashima K, Terakado H, Yamamoto N. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol, 23:382-388, 2018
4. Hosaka M, Inoue R, Satoh M, Watabe D, Hanazawa T, Ohkubo T, Asayama K, Obara T, Imai Y. Effect of amlodipine, efonidipine, and trichlormethiazide on home blood pressure and upper-normal microalbuminuria assessed by casual spot urine test in essential hypertensive patients. Clin Exp Hypertens, 40:468-475, 2018
5. Nakashima T, Koido K, Baba H, Otsuka R, Okinaka K, Sano T, Nishigaki R, Hashimoto H, Otsuka T, Esaki M, Terakado H. Contribution of pharmacists with expertise in infectious diseases to appropriate individualized vancomycin dosing. Pharmazie, 73:422-424, 2018
6. Shimizu H, Suzuki K, Uchikura T, Tsuji D, Yamanaka T, Hashimoto H, Goto K, Matsui R, Seki N, Shimada T, Ikeda S, Ikegami N, Hama T, Yamamoto N, Sasaki T. Economic analysis of palonosetron versus granisetron in the standard triplet regimen for preventing chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy in Japan (TRIPLE phase III trial). J Pharm Health Care Sci, 4:31, 2018
7. Watabe D, Asayama K, Hanazawa T, Hosaka M, Satoh M, Yasui D, Obara T, Inoue R, Metoki H, Kikuya M, Imai Y, Ohkubo T. Predictive power of home blood pressure indices at baseline and during follow-up in hypertensive patients: HOMED-BP study. Hypertens Res, 41:622-628, 2018
8. Hanazawa T, Asayama K, Watabe D, Tanabe A, Satoh M, Inoue R, Hara A, Obara T, Kikuya M, Nomura K, Metoki H, Imai Y, Ohkubo T. Association Between Amplitude of Seasonal Variation in Self-Measured Home Blood Pressure and Cardiovascular Outcomes: HOMED-BP (Hypertension Objective Treatment Based on Measurement By Electrical Devices of Blood Pressure) Study. J Am Heart Assoc, 7:2018
9. Saito Y, Kumamoto T, Shiraiwa M, Sonoda T, Arakawa A, Hashimoto H, Tamai I, Ogawa C, Terakado H. Cyclophosphamide-induced hemorrhagic cystitis in young patients with solid tumors: A single institution study. Asia Pac J Clin Oncol, 14:e460-e464, 2018
10. Arai S, Hara T, Matsui Y, Koido K, Hashimoto H, Shinoda Y, Komiyama M, Fujimoto H, Terakado H. Tolerability and Efficacy of Neoadjuvant Chemotherapy with a Tri-Weekly Interval Methotrexate, Doxorubicin, Vinblastine, and Cisplatin Regimen for Patients with Locally Advanced Bladder Cancer. Case Rep Oncol, 11:450- 460, 2018
11. Hashimoto H, Abe M, Yanai T, Yamaguchi T, Zenda S, Uchitomi Y, Fukuda H, Mori M, Iwasa S, Yamamoto N, Ohe Y. Study protocol for J-SUPPORT 1604 (J-FORCE): a randomized, double blind, placebo-controlled Phase III study evaluating olanzapine (5 mg) plus standard triple antiemetic therapy for prevention of chemotherapy induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy. Jpn J Clin Oncol, 48:950-952, 2018
