Annual Report 2019
Department of Breast and Medical Oncology
Toru Mukohara, Ako Hosono, Yoichi Naito, Nobuaki Matsubara, Hirofumi Mukai, Takahiro Kogawa, and Kenichi Harano, Yoko Fukasawa, Shota Kusuhara, Masako Inoue, Yoriko Hasegawa, Yumi Fujimoto
Introduction
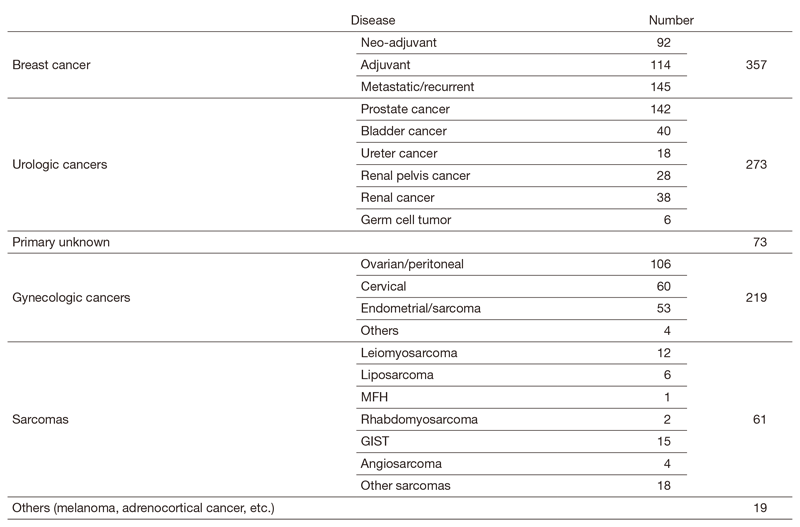
In clinic, we are taking care of patients with breast, urologic, gynecologic, and primary-unknown cancers, and sarcoma. We are also in charge of rare cancers that are difficult to be treated at other hospitals because of the absence of standard therapy.
We contributed to the operation of the “Lady’s center” that opened in 2018 as a core of multi-disciplinary care for women with cancer. We also played central roles in implementing multi-gene panel testing, which has come under insurance coverage in 2019. Further, we ran the Hereditary Breast and Ovarian Cancer syndrome (HBOC) clinic to provide proper information to patients with a high risk of the syndrome. We also developed an institutional system for upcoming insurance coverage of BRCA genetic testing, and associated Risk Reducing Mastectomy (RRM) and Risk Reducing Salpingo-Oophorectomy (RRSO).
Research activities
We are participating in many company-sponsored trials and other collaborative studies of clinical trial groups such as JCOG, JBCRG, and WJOG. Our contribution to WJOG studies was recognized and we received the Ariyoshi-Fukuoka Award 2019.
We are also running collaborative studies with a technology company to explore the clinical utility of their system for detecting circulating tumor cells (CTCs).
We are also implementing clinical and translational research with funds obtained from Grant-in-Aid for Scientific Research (“3D co-culture of cancer cells in malignant effusion / CTC with adipose stem cell”; PI, Mukohara T) (“New sub-classification of triple negative breast cancer to evaluate immunogenicity”; PI, Kogawa T). This year, we ran a preclinical project about combination therapy for HER2-positive breast cancer in a collaboration with the Division of Genome TR.
Education
Our goal in education is fostering “genuine” medical oncologist. Graduates from our Resident / Chief Resident programs are expected to be capable of providing not only standard therapies regardless of types of cancer but also multi-disciplinary care cooperating with other medical professionals. Obtaining skills for palliative care and dealing with oncologic emergencies are also expected. Further, we require them and help them to implement clinical research to address clinical questions they find for themselves and report the results in internal journals. That is because we want to develop scientific clinicians who can focus on issues scientifically and create evidence for themselves. This year, one graduate from our residency program earned Diplomate, Subspecialty Board of Medical Oncology from JSMO.
Further, we were awarded an educational grant with the theme “Comprehensive educational program to develop medical professionals and peer supporters to empower Adolescent and Young Adult (AYA) breast cancer patients”. We formed a multidisciplinary AYA conference (mAYAcon) to deliver optimal care to AYA cancer patients and to conduct educational programs for medical professionals.
Future prospects
In the clinic, we will provide clinical care with high patient satisfaction through a multi-disciplinary team approach. In education, we will foster medical oncologists who have skill and knowledge for cross-organ oncology care and scientific acumen. In research, we will initiate multi-center clinical trials and phase I to III developmental trials of investigational drugs. We will also expand our research activity to preclinical research for response predictions and overcoming resistance for anti-cancer drugs. Multiple research themes are currently under discussion with industries and other academic organizations.
List of papers published in 2019
Journal
1. Doi T, Matsubara N, Kawai A, Naka N, Takahashi S, Uemura H, Yamamoto N. Phase I study of TAS-115, a novel oral multi-kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs, 38:1175-1185, 2020
2. Naito Y, Mishima S, Akagi K, Igarashi A, Ikeda M, Okano S, Kato S, Takano T, Tsuchihara K, Terashima K, Nishihara H, Nishiyama H, Hiyama E, Hirasawa A, Hosoi H, Maeda O, Yatabe Y, Okamoto W, Ono S, Kajiyama H, Nagashima F, Hatanaka Y, Miyachi M, Kodera Y, Yoshino T, Taniguchi H. Japan society of clinical oncology/Japanese society of medical oncology-led clinical recommendations on the diagnosis and use of tropomyosin receptor kinase inhibitors in adult and pediatric patients with neurotrophic receptor tyrosine kinase fusion-positive advanced solid tumors, cooperated by the Japanese society of pediatric hematology/oncology. Int J Clin Oncol, 25:403-417, 2020
3. Kogawa T, Fujii T, Wu J, Harano K, Fouad TM, Liu DD, Shen Y, Masuda H, Krishnamurthy S, Chavez-MacGregor M, Lim B, Murthy RK, Valero V, Tripathy D, Ueno NT. Prognostic Value of HER2 to CEP17 Ratio on Fluorescence In Situ Hybridization Ratio in Patients with Nonmetastatic HER2-Positive Inflammatory and Noninflammatory Breast Cancer Treated with Neoadjuvant Chemotherapy with or without Trastuzumab. Oncologist, 25:e909-e919, 2020
4. Kawazoe A, Kuboki Y, Bando H, Fukuoka S, Kojima T, Naito Y, Iino S, Yodo Y, Doi T, Shitara K, Yoshino T. Phase 1 study of napabucasin, a cancer stemness inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol, 85:855-862, 2020
5. Matsubara N, Suzuki K, Kazama H, Tsukube S, Seto T, Matsuyama H. Cabazitaxel in patients aged ≧80 years with castration-resistant prostate cancer: Results of a post-marketing surveillance study in Japan. J Geriatr Oncol, S1879-4068(19)30502-8, 2020
6. Suzuki H, Shin T, Fukasawa S, Hashine K, Kitani S, Ohtake N, Shibayama K, Tran N, Mundle S, Fizazi K, Matsubara N. Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naive prostate cancer: final subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, phase 3 study. Jpn J Clin Oncol, 50:810-820, 2020
7. Tsuda H. Histological classification of breast tumors in the General Rules for Clinical and Pathological Recording of Breast Cancer (18th edition). Breast Cancer, 27:309-321, 2020
8. Kato H, de Souza P, Kim SW, Lickliter JD, Naito Y, Park K, Kumar S, Mugundu GM, Bang YJ. Safety, Pharmacokinetics, and Clinical Activity of Adavosertib in Combination with Chemotherapy in Asian Patients with Advanced Solid Tumors: Phase Ib Study. Target Oncol, 15:75-84, 2020
9. Masuda T, Noda M, Kogawa T, Kitagawa D, Hayashi N, Jomori T, Nakanishi Y, Nakayama KI, Ohno S, Mimori K. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci, 111:924-931, 2020
10. Kobayashi T, Terada N, Kimura T, Matsubara N, Murakami K, Mori K, Fujimoto Y, Akamatsu S, Inoue T, Ogawa O. Sequential Use of Androgen Receptor Axis-targeted Agents in Chemotherapy-naive Castration-resistant Prostate Cancer: A Multicenter Retrospective Analysis With 3-Year Follow-up. Clin Genitourin Cancer, 18:e46-e54, 2020
11. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med, 382:610-621, 2020
12. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O'Shaughnessy J. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med, 382:810-821, 2020
13. Niikura N, Nakatukasa K, Amemiya T, Watanabe KI, Hata H, Kikawa Y, Taniike N, Yamanaka T, Mitsunaga S, Nakagami K, Adachi M, Kondo N, Shibuya Y, Hayashi N, Naito M, Kashiwabara K, Yamashita T, Umeda M, Mukai H, Ota Y. Oral Care Evaluation to Prevent Oral Mucositis in Estrogen Receptor-Positive Metastatic Breast Cancer Patients Treated with Everolimus (Oral Care-BC): A Randomized Controlled Phase III Trial. Oncologist, 25:e223-e230, 2020
14. Naito Y, Kai Y, Ishikawa T, Fujita T, Uehara K, Doihara H, Tokunaga S, Shimokawa M, Ito Y, Saeki T. Chemotherapy-induced nausea and vomiting in patients with breast cancer: a prospective cohort study. Breast Cancer, 27:122-128, 2020
15. Petrylak DP, de Wit R, Chi KN, Drakaki A, Sternberg CN, Nishiyama H, Castellano D, Hussain SA, Fléchon A, Bamias A, Yu EY, van der Heijden MS, Matsubara N, Alekseev B, Necchi A, Géczi L, Ou YC, Coskun HS, Su WP, Bedke J, Gakis G, Percent IJ, Lee JL, Tucci M, Semenov A, Laestadius F, Peer A, Tortora G, Safina S, Garcia Del Muro X, Rodriguez-Vida A, Cicin I, Harputluoglu H, Tagawa ST, Vaishampayan U, Aragon-Ching JB, Hamid O, Liepa AM, Wijayawardana S, Russo F, Walgren RA, Zimmermann AH, Hozak RR, Bell-McGuinn KM, Powles T. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): overall survival and updated results of a randomised, double-blind, phase 3 trial. Lancet Oncol, 21:105-120, 2020
16. Nishida T, Sakai Y, Takagi M, Ozaka M, Kitagawa Y, Kurokawa Y, Masuzawa T, Naito Y, Kagimura T, Hirota S. Adherence to the guidelines and the pathological diagnosis of high-risk gastrointestinal stromal tumors in the real world. Gastric Cancer, 23:118-125, 2020
17. Inoue K, Takahashi M, Mukai H, Yamanaka T, Egawa C, Sakata Y, Ikezawa H, Matsuoka T, Tsurutani J. Effectiveness and safety of eribulin in Japanese patients with HER2-negative, advanced breast cancer: a 2-year post-marketing observational study in a real-world setting. Invest New Drugs, 2020
18. Rugo HS, Finn RS, Gelmon K, Joy AA, Harbeck N, Castrellon A, Mukai H, Walshe JM, Mori A, Gauthier E, Lu DR, Bananis E, Martin M, Diéras V. Progression-free Survival Outcome Is Independent of Objective Response in Patients With Estrogen Receptor-positive, Human Epidermal Growth Factor Receptor 2-negative Advanced Breast Cancer Treated With Palbociclib Plus Letrozole Compared With Letrozole: Analysis From PALOMA-2. Clin Breast Cancer, 20:e173-e180, 2020
19. Kobayashi E, Naito Y, Asano N, Maejima A, Endo M, Takahashi S, Megumi Y, Kawai A. Interim results of a real-world observational study of eribulin in soft tissue sarcoma including rare subtypes. Jpn J Clin Oncol, 49:938-946, 2019
20. Doi T, Fujiwara Y, Matsubara N, Tomomatsu J, Iwasa S, Tanaka A, Endo-Tsukude C, Nakagawa S, Takahashi S. Phase I study of ipatasertib as a single agent and in combination with abiraterone plus prednisolone in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 84:393-404, 2019
21. Goto K, Fujiwara Y, Isobe T, Chayahara N, Kiyota N, Mukohara T, Tsubata Y, Hotta T, Tamura K, Yamamoto N, Minami H. Pharmacokinetic study of the oral fluorouracil antitumor agent S-1 in patients with impaired renal function. Cancer Sci, 110:1987-1994, 2019
22. Shimomura A, Yonemori K, Yoshida M, Yoshida T, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hamada A, Michimae H, Hashimoto J, Yamamoto H, Kawachi A, Shimizu C, Fujiwara Y, Tamura K. Gene Alterations in Triple-Negative Breast Cancer Patients in a Phase I/II Study of Eribulin and Olaparib Combination Therapy. Transl Oncol, 12:1386-1394, 2019
23. Esaki T, Hirai F, Makiyama A, Seto T, Bando H, Naito Y, Yoh K, Ishihara K, Kakizume T, Natsume K, Myers A, Doi T. Phase I dose-escalation study of capmatinib (INC280) in Japanese patients with advanced solid tumors. Cancer Sci, 110:1340-1351, 2019
24. Doi T, Kurokawa Y, Sawaki A, Komatsu Y, Ozaka M, Takahashi T, Naito Y, Ohkubo S, Nishida T. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: a phase II, single-arm trial. Eur J Cancer, 121:29-39, 2019
25. Suzuki K, Matsubara N, Kazama H, Seto T, Tsukube S, Matsuyama H. Safety and efficacy of cabazitaxel in 660 patients with metastatic castration-resistant prostate cancer in real-world settings: results of a Japanese post-marketing surveillance study. Jpn J Clin Oncol, 49:1157-1163, 2019
26. Akazawa Y, Hosono A, Yoshikawa T, Kaneda H, Nitani C, Hara J, Kinoshita Y, Kohashi K, Manabe A, Fukutani M, Wakabayashi M, Sato A, Shoda K, Shimomura M, Mizuno S, Nakamoto Y, Nakatsura T. Efficacy of the NCCV Cocktail-1 vaccine for refractory pediatric solid tumors: A phase I clinical trial. Cancer Sci, 110:3650-3662, 2019
27. Miyachi M, Tsuchiya K, Hosono A, Ogawa A, Koh K, Kikuta A, Hara J, Teramukai S, Hosoi H. Phase II study of vincristine, actinomycin-D, cyclophosphamide and irinotecan for patients with newly diagnosed low-risk subset B rhabdomyosarcoma: A study protocol. Medicine (Baltimore), 98:e18344, 2019
28. Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, Ueno T, Kwong A, Li H, Huang SM, Leung R, Han W, Tan B, Hu FC, Huang CS, Cheng AL, Lu YS. Contrasting Epidemiology and Clinicopathology of Female Breast Cancer in Asians vs the US Population. J Natl Cancer Inst, 111:1298-1306, 2019
29. Yeo W, Ueno T, Lin CH, Liu Q, Lee KH, Leung R, Naito Y, Park YH, Im SA, Li H, Yap YS, Lu YS. Treating HR+/HER2- breast cancer in premenopausal Asian women: Asian Breast Cancer Cooperative Group 2019 Consensus and position on ovarian suppression. Breast Cancer Res Treat, 177:549-559, 2019
30. Tamura K, Hasegawa K, Katsumata N, Matsumoto K, Mukai H, Takahashi S, Nomura H, Minami H. Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial. Cancer Sci, 110:2894-2904, 2019
31. Masuda N, Mukai H, Inoue K, Rai Y, Ohno S, Mori Y, Hashigaki S, Muramatsu Y, Umeyama Y, Iwata H, Toi M. Correction to: Neutropenia management with palbociclib in Japanese patients with advanced breast cancer. Breast Cancer, 26:651, 2019
32. Masuda N, Mukai H, Inoue K, Rai Y, Ohno S, Mori Y, Hashigaki S, Muramatsu Y, Umeyama Y, Iwata H, Toi M. Neutropenia management with palbociclib in Japanese patients with advanced breast cancer. Breast Cancer, 26:637-650, 2019
33. Ohno S, Mukai H, Narui K, Hozumi Y, Miyoshi Y, Yoshino H, Doihara H, Suto A, Tamura M, Morimoto T, Zaha H, Chishima T, Nishimura R, Ishikawa T, Uemura Y, Ohashi Y. Participants in a randomized controlled trial had longer overall survival than non-participants: a prospective cohort study. Breast Cancer Res Treat, 176:631-635, 2019
34. Chayahara N, Mukohara T, Tachihara M, Fujishima Y, Fukunaga A, Washio K, Yamamoto M, Nakata K, Kobayashi K, Takenaka K, Toyoda M, Kiyota N, Tobimatsu K, Doi H, Mizuta N, Marugami N, Kawaguchi A, Nishigori C, Nishimura Y, Minami H. Adapalene Gel 0.1% Versus Placebo as Prophylaxis for Anti-Epidermal Growth Factor Receptor-Induced Acne-Like Rash: A Randomized Left-Right Comparative Evaluation (APPEARANCE). Oncologist, 24:885-e413, 2019
35. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, Sulur G, Luna Y, Li S, Mundle S, Chi KN. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol, 20:686-700, 2019
36. Uemura H, Uemura H, Nagamori S, Wakumoto Y, Kimura G, Kikukawa H, Yokomizo A, Mizokami A, Kosaka T, Masumori N, Kawasaki Y, Yonese J, Nasu Y, Fukasawa S, Sugiyama T, Kinuya S, Hosono M, Yamaguchi I, Akagawa T, Matsubara N. Three-year follow-up of a phase II study of radium-223 dichloride in Japanese patients with symptomatic castration-resistant prostate cancer and bone metastases. Int J Clin Oncol, 24:557-566, 2019
37. Im SA, Mukai H, Park IH, Masuda N, Shimizu C, Kim SB, Im YH, Ohtani S, Huang Bartlett C, Lu DR, Iyer S, Mori Y, Mori A, Gauthier E, Finn RS, Toi M. Palbociclib Plus Letrozole as First-Line Therapy in Postmenopausal Asian Women With Metastatic Breast Cancer: Results From the Phase III, Randomized PALOMA-2 Study. J Glob Oncol, 5:1-19, 2019
38. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37-298 women with early breast cancer in 26 randomised trials. Lancet, 393:1440-1452, 2019
39. Masuda N, Inoue K, Nakamura R, Rai Y, Mukai H, Ohno S, Hara F, Mori Y, Hashigaki S, Muramatsu Y, Nagasawa T, Umeyama Y, Huang X, Iwata H. Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int J Clin Oncol, 24:262-273, 2019
