Annual Report 2022
Department of Thoracic Surgery
Masahiro Tsuboi, Keiju Aokage, Joji Samejima, Tomohiro Miyoshi, Kenta Tane, Yutaro Koike
Introduction
The Department of Thoracic Surgery has three missions: surgical treatment, surgical resident training, and clinical research. Thoracic surgeries involve the treatment of thoracic neoplasms, primary and metastatic lung tumors, as well as mediastinal, pleural, and chest wall tumors. Our department specializes in the surgical treatment of pulmonary carcinomas. Routine surgical treatment modalities for carcinomas include limited resection (wedge or segmental resection) and simple resection (lobectomy or pneumonectomy), with or without systematic lymph node dissection. Thoracoscopic assistance is almost always used. Robotic-assisted thoracic surgery and single-port thoracoscopic surgery are also performed. Non-routine surgical procedures involve complex approaches such as broncho-/angio-plasty, combined resection with adjacent structures, and perioperative adjuvant treatment. Since its establishment in 1992, our department has been one of the most active leaders in the field of lung cancer in Japan. Moreover, it has actively participated in international and national scientific venues. This year, in addition to 16 scientific papers published in English, our department made 30 presentations: four international, 23 national, and four regional.
The Team and What We Do
The Department of Thoracic Surgery is presently composed of four consultant surgeons including a chief and eight or nine residents. Our department has adopted a team approach to patient treatment and resident training. Potential surgical intervention candidate cases are presented every Tuesday evening at a multidisciplinary team conference of thoracic surgeons, oncology physicians, radiologists, and residents.
Each case is thoroughly and vigorously reviewed and discussed. Treatment modality discussions are conducted in English to improve the English fluency of staff members and residents in preparation for international presentations and to better involve visiting physicians from other countries. Moreover, selected patients’ records are radiologically and cytopathologically reviewed every Friday morning. These reviews aim to improve the interpretation of radiologic indications to pathology findings, accurately evaluate surgical indications, and upgrade knowledge on rare histology. Case review meetings are held twice a week by a multidisciplinary team, with resectable or borderline resectable diseases on Tuesdays and unresectable or metastatic diseases on Wednesdays. We believe that these activities improve the knowledge base, treatment indications, and surgical treatment. For non-small cell histology, primary pulmonary carcinomas in clinical stages I/II and IIIA without bulky or multistation-involved mediastinal nodes, and primary pulmonary small cell carcinomas in clinical stage I, surgical resection is indicated for cure. Optimum treatment modalities are being sought via clinical trials to improve the poor prognosis of patients with bulky or clinically and histologically proven multistation mediastinal lymph node metastases, with disease invading the neighboring vital structures, or with small cell cancers in clinical stage II and later. Resection of metastatic lung tumor is attempted based on modified Thomfold’s criteria after patient consultation. The majority of these cases are metastases from colorectal carcinomas, while most of the mediastinal tumors are thymic epithelial tumors. The surgical procedures of the Department of Thoracic Surgery have generally remained similar for the past decade. Still, we have employed port-access thoracoscopic surgery more often for the last several years. Approximately 20% of the surgeries are completed via a 3-port access, and 70% are video-thoracoscopically assisted. To date, the average postoperative hospital stay of patients in our department has improved and become shorter, three days being the shortest, with a median of seven days for primary lung cancer cases. These shorter hospital stays are achieved with a slightly better complication rate than normal. This year, 30-day operative mortality occurred in two patients undergoing surgery for primary lung cancer.
Research Activities
The mission of our research is to cure as many patients with lung cancer or thoracic malignancies as possible while maintaining their individual quality of life. To achieve this, we try to do what only the National Cancer Center can do, and we take the lead in connecting to multi-center or multi-national research activities.
Research in the area of combined treatment, especially immunotherapy and molecular targeted therapy, has now advanced to clinical trials. Some of these treatments have been approved and reimbursed as standard treatments. It is the goal for researchers in our department to acquire a basic understanding of the cellular and molecular mechanisms leading to the development and progression of lung cancer and apply these findings to further the development of immunotherapy-based prevention and treatment strategies.
Recently, in addition to research on surgical techniques and perioperative care, we are conducting research that will lead to the development of medical devices.
Clinical Trials
There are the following prospective trials in this department.
1. Primary investigator and a member of an organized trial of sublobar resection for peripheral GGO dominant cT1aN0M0 lung adenocarcinomas [JCOG 0804, phase II, the primary endpoint: RFS was published in JTCVS.].
2. Study coordinator and a member of an organized trial of segmental resection vs. lobectomy for peripheral T1aN0M0 non-small cell lung cancers [JCOG 0802, phase III. The OS and RFS data were presented in the 101st AATS, and published in Lancet].
3. Study coordinator and a member of an organized trial of sublobar resection for peripheral GGO dominant cT1bN0M0 lung adenocarcinomas [JCOG 1211, phase III, the RFS data were presented in the 35th EATCS and published in Lancet Resp. Med.]
4. Primary investigator and a member of an organized trial of Cisplatin/Pemetrexed vs. Cisplatin/Vinorelbine adjuvant chemotherapy for completely resected pathologic stage II-IIIA non-small cell lung cancer [JIPANG, phase III, patient accrual was completed. The primary endpoint: DFS and a key secondary endpoint: OS were reported in JCO].
5. Study coordinator and a member of an organized trial of a randomized controlled trial of the therapeutic value of lobe-selective lymph node dissection for clinical stage I/II Non-Small Cell Lung Cancer. [L-SEPC trial, phase III, patient accrual is ongoing]
6. Study coordinator and a member of an organized trial of a single-arm validation study of follow-up for early-stage lung cancer based on thin-section CT findings of the chest. [JCOG1906: Ever Green trial, phase II, patient accrual is ongoing]
7. Primary investigator and a member of an organized trial of a Single-Arm Validation Study of Multimodality Treatment of Chest Wall Invasive Cancer of the Superior Sulcus in the lung with Pre- and Postoperative Durvalumab or Durvalumab Maintenance Treatment after Chemoradiotherapy. [JCOG1807C: Deep Ocean trial, phase II, patient accrual is ongoing]
8. Study coordinator and a member of an organized trial of a Randomized Controlled Trial of Postoperative Surveillance for Pathologic Stage II-IIIA Non-Small Cell Lung Cancer. [JCOG2012: Phoenix trial, phase III, patient accrual is ongoing]
9. Primary investigator and a member of an organized trial of Induction Therapy with pembrolizumab + ramucirumab and Surgery as Multimodality Treatment for PD-L1-Positive Stage IB-IIIA Non-Small Cell Lung Cancer. [East Energy, Investigator-initiated trial, phase II, the MPR data were presented in ASCO2023]
In addition, we are conducting many corporate trials for the development of perioperative treatment using a molecular targeted drugs (osimertinib) and immune checkpoint inhibitors (nivolumab, pembrolizumab, atezolizumab), and a corporate trial for the development of sealants for intraoperative pulmonary fistulas.
Education
Our training program aims to educate residents by expanding their knowledge and technical skills in treating lung cancer, other thoracic malignancies, and benign tumors, such as hamartoma and mediastinal cystic lesions. In addition, we seek to instill in the trainee a desire for continued introspection and self-education, open communication between all healthcare providers, while maintaining a respectful and professional demeanor. (Table 1, Table 2)
Table 1. Number of patients with thoracic surgery 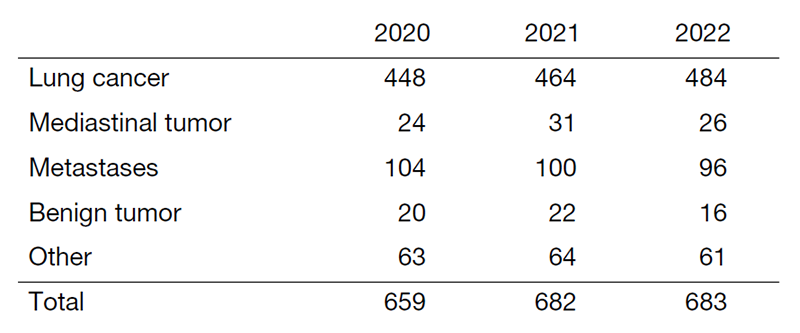

Table 2. Type of procedure in surgical cases with lung cancer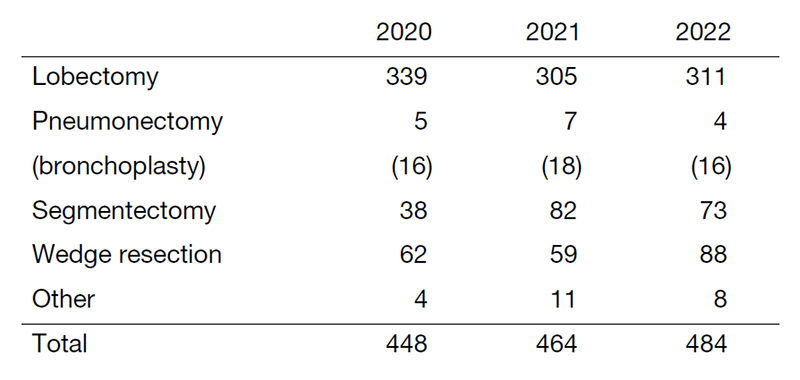

Future Prospects
The treatment of thoracic malignancies, including lung cancer, mesothelioma, thymic malignancies, and lung metastases, has made steady progress with the development of molecular targeted drugs and immune checkpoint inhibitors. Still, the situation in perioperative treatment remains exploratory. We are also continuing research and development on surgical techniques, taking into consideration both oncological aspects and invasiveness. Coordination of our laboratory's activities with such clinical research is essential for the advancement of treatment and improvement of cure rates after surgery. We will continue to focus on delivering effective treatments to patients with thoracic cancer as soon as possible, in cooperation with researchers and companies in Japan and around the world.
List of papers published in 2022
Journal
1. Aokage K, Suzuki K, Saji H, Wakabayashi M, Kataoka T, Sekino Y, Fukuda H, Endo M, Hattori A, Mimae T, Miyoshi T, Isaka M, Yoshioka H, Nakajima R, Nakagawa K, Okami J, Ito H, Kuroda H, Tsuboi M, Okumura N, Takahama M, Ohde Y, Aoki T, Tsutani Y, Okada M, Watanabe SI. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. The Lancet. Respiratory medicine, 11:540-549, 2023
2. Sakai T, Aokage K, Miyoshi T, Tane K, Ishii G, Goto K, Tsuboi M. Tumor size exceeding 5 cm as a valid prognostic factor in all stages of thymic epithelial tumors. Surgery today, 53:42-50, 2023
3. Onodera K, Suzuki J, Miyoshi T, Tane K, Samejima J, Aokage K, Tsuboi M. Comparison of various lung intersegmental plane identification methods. General thoracic and cardiovascular surgery, 71:90-97, 2023
4. Konishi Y, Taki T, Nakai T, Kuroe T, Morisue R, Miyoshi T, Tane K, Samejima J, Aokage K, Miyazaki S, Sakamoto N, Sakashita S, Watanabe R, Kojima M, Suzuki K, Tsuboi M, Ishii G. Clinicopathological features and prognostic impact of dirty necrosis in metastatic lung cancers from the colon and rectum. Cancer science, 114:2169-2177, 2023
5. Kamigaichi A, Aokage K, Ikeno T, Wakabayashi M, Miyoshi T, Tane K, Samejima J, Tsuboi M. Long-term survival outcomes after lobe-specific nodal dissection in patients with early non-small-cell lung cancer. European journal of cardio-thoracic surgery, 63:ezad016, 2023
6. Aokage K, Tsuboi M, Zenke Y, Horinouchi H, Nakamura N, Ishikura S, Nishikawa H, Kumagai S, Koyama S, Kanato K, Kataoka T, Wakabayashi M, Fukutani M, Fukuda H, Ohe Y, Watanabe SI. Study protocol for JCOG1807C (DEEP OCEAN): a interventional prospective trial to evaluate the efficacy and safety of durvalumab before and after operation or durvalumab as maintenance therapy after chemoradiotherapy against superior sulcus non-small cell lung cancer. Japanese journal of clinical oncology, 52:383-387, 2022
7. Nomura K, Nakai T, Nishina Y, Sakamoto N, Miyoshi T, Tane K, Samejima J, Aokage K, Kojima M, Sakashita S, Taki T, Miyazaki S, Watanabe R, Suzuki K, Tsuboi M, Ishii G. 18F-fluorodeoxyglucose uptake in PET is associated with the tumor microenvironment in metastatic lymph nodes and prognosis in N2 lung adenocarcinoma. Cancer science, 113:1488-1496, 2022
8. Niimi T, Nakai T, Aokage K, Tane K, Miyoshi T, Samejima J, Miyazaki S, Taki T, Sakamoto N, Sakashita S, Watanabe R, Kojima M, Suzuki K, Tsuboi M, Ishii G. Prognostic impact of count of extratumoral lymphatic permeation in lung adenocarcinoma and its relation to the immune microenvironment. Cancer science, 113:1497-1506, 2022
9. Noritake O, Aokage K, Suzuki A, Tane K, Miyoshi T, Samejima J, Yoshikawa T, Murata SC, Nakai T, Tsuboi M, Ishii G. Prognostic impact of the number of peri-tumoral alveolar macrophages in patients with stage I lung adenocarcinoma. Journal of cancer research and clinical oncology, 148:3437-3447, 2022
10. Kamigaichi A, Aokage K, Katsumata S, Ishii G, Wakabayashi M, Miyoshi T, Tane K, Samejima J, Tsuboi M. Prognostic impact of examined mediastinal lymph node count in clinical N0 non-small cell lung cancer. European journal of cardio-thoracic surgery, 62:ezac359, 2022
