Annual Report 2022
Department of Medical Oncology
Kan Yonemori, Tatsunori Shimoi, Kazuki Sudo, Tadaaki Nishikawa, Yuki Kojima, Aiko Maejima, Kasumi Yamamoto, Shosuke Kita, Ayumi Saito, Motoko Arakaki, Eijiro Nakamura, Hitomi Sumiyoshi-Okuma, Asuka Kawachi, Shu Yazaki, Momoko Tokura, Rui Kitadai, Yasuhiro Fujiwara, Emi Noguchi
Introduction
The Department of Medical Oncology provides the most effective treatments by the use of chemotherapy, and works on the establishment of new standards of care for adult malignancies including breast cancer, gynecologic cancer, urological cancer, soft-tissue sarcoma, extra-gonadal germ cell tumor, cancer of unknown primary site and other rare types of solid tumors.
We envision becoming a leading medical oncology department, which makes a difference in cancer care in Japan and worldwide. Our mission is to provide patient-centered, state-of-the-art medical care to cancer patients, to develop new effective cancer treatments through clinical and translational research, and to nurture medical oncologists. An evidence-based, research-oriented and multidisciplinary approach is the core value of our practice.
The Team and What We Do
The Department of Medical Oncology conducts clinical, research and educational activities with a focus on pharmacotherapy for breast, uterine, ovarian, urological cancers, malignant soft tissue tumors (e.g. sarcomas), germ cell tumors, cancers of unknown primary, and other rare malignancies (Table 1).
The five basic missions are as follows:
- Contribute to the development of new cancer therapies domestically and globally by planning, supervising or participating in clinical trials.
- Promote translational research and strengthen cooperation with other departments, professions and research centers.
- Lead the world in the development of treatments for breast cancers, gynecological cancers, urological cancers and sarcoma, and in the early clinical development of anti-cancer drugs.
- Educate and train specialists in cancer pharmacotherapy or breast cancer treatment (medical specialists).
- Understand the physical, mental and social suffering of cancer patients and aim for holistic medical care.
Table 1. 1st Visiting Patients to the Department of Medical Oncology (April 2020 to March 2021)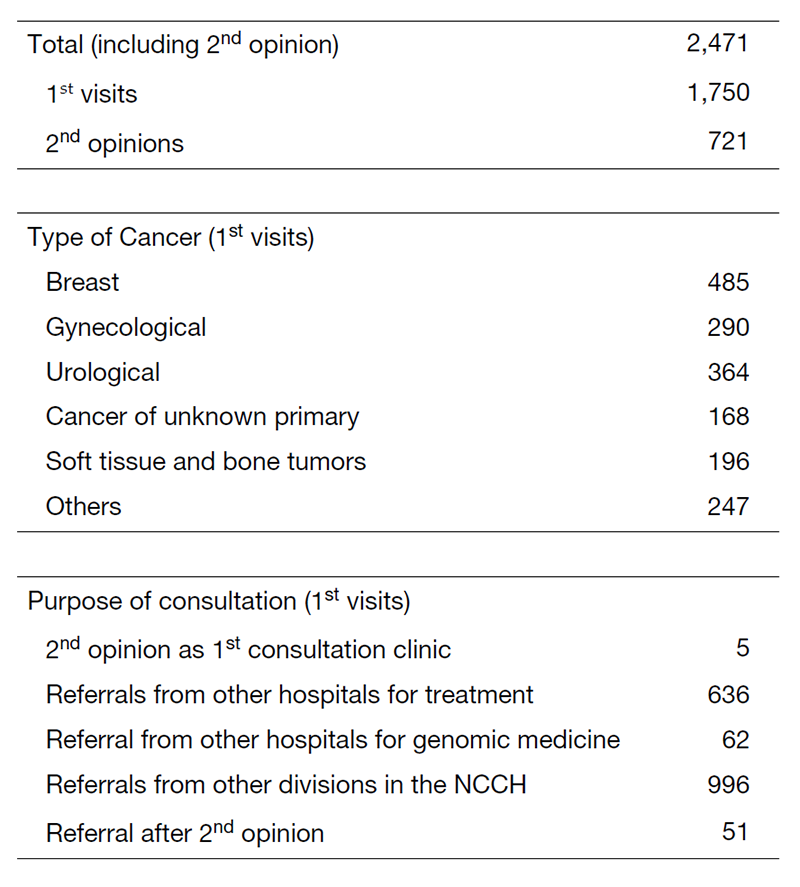

In addition to providing the latest evidence-based pharmacotherapy, the department focuses on research activities aimed at building new evidence, focusing on the social environment surrounding cancer care, and developing treatments for rare cancers.
Research Activities
Our research interests extend across a wide range of topics related to treatment and clinical program development. Many of our research programs are secured by public and consignment research grants. In fiscal year 2022, we conducted many clinical studies as a primary investigator and participated in additional programs as a co-investigator in research programs secured by competitive public research funds.
We published 84 international manuscripts, focusing on early-phase anti-cancer drug development, molecular imaging, drug efficacy studies using patient derived xenografts, translational research, novel chemotherapy against breast and gynecological cancer, novel biomarkers to predict the efficacy and adverse events of anti-cancer drugs and other basic researches. We value cancer survivorship as a research theme to develop a comprehensive patient-centered care program.
Clinical Trials
In fiscal year 2022, we actively enrolled patients in phase I studies (including first in human or global) as well as domestic and international phase II and III studies (Table 2). Of note we enrolled patients in IIT for rare cancers (IIT in Table 2). We also conducted many types of translational studies (TR) to identify novel biomarkers.
Education
We provide rich educational opportunities to both residents and chief residents through clinical experience as well as research activities. Residents are encouraged to make presentations at local and national conferences. We vigorously support basic, clinical, and translational research conducted by postdoctoral researchers.
Future Prospects
We will continue to establish new standard treatments and propose a near-future model for the clinical management of adult solid tumors, including breast cancer, and gynecologic cancer. Moreover, we aim to build a comprehensive program, which includes tumor registry, translational research, clinical trials and patient care in rare adult tumors based on our rich clinical experience. We would also like to improve the efficiency of anti-cancer drug development by coordinating basic and translational research in early-phase clinical trials.
Table 2. Active Clinical Trials (April 2022 to March 2023)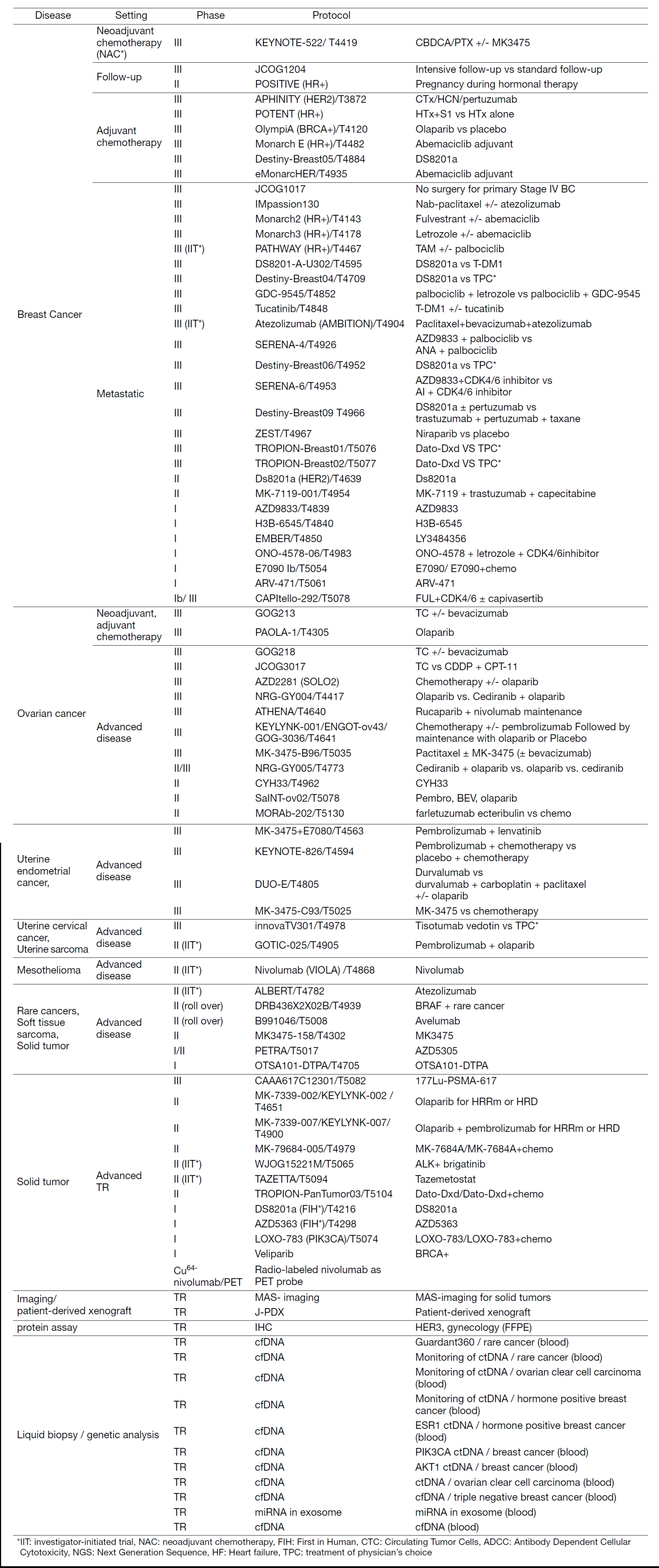

List of papers published in 2022
Journal
1. Eguchi K, Omura G, Shimoi T, Kageyama D, Igaki H, Abe Y, Watanabe T, Aihara Y, Sakai A, Matsumoto Y, Sakai T, Yonemori K, Mori T, Yoshida A, Yoshimoto S. BCOR-CCNB3 sarcoma arising in the pharynx. Auris, nasus, larynx, 50:618-622, 2023
2. Yazaki S, Shimoi T, Yoshida M, Sumiyoshi-Okuma H, Arakaki M, Saito A, Kita S, Yamamoto K, Kojima Y, Nishikawa T, Tanioka M, Sudo K, Noguchi E, Murata T, Shiino S, Takayama S, Suto A, Ohe Y, Fujiwara Y, Yonemori K. Integrative prognostic analysis of tumor-infiltrating lymphocytes, CD8, CD20, programmed cell death-ligand 1, and tertiary lymphoid structures in patients with early-stage triple-negative breast cancer who did not receive adjuvant chemotherapy. Breast cancer research and treatment, 197:287-297, 2023
3. Yazaki S, Salgado R, Shimoi T, Yoshida M, Shiino S, Kaneda T, Kojima Y, Sumiyoshi-Okuma H, Nishikawa T, Sudo K, Noguchi E, Murata T, Takayama S, Suto A, Ohe Y, Yonemori K. Impact of adjuvant chemotherapy and radiotherapy on tumour-infiltrating lymphocytes and PD-L1 expression in metastatic breast cancer. British journal of cancer, 128:568-575, 2023
4. Matsuzaki J, Kato K, Oono K, Tsuchiya N, Sudo K, Shimomura A, Tamura K, Shiino S, Kinoshita T, Daiko H, Wada T, Katai H, Ochiai H, Kanemitsu Y, Takamaru H, Abe S, Saito Y, Boku N, Kondo S, Ueno H, Okusaka T, Shimada K, Ohe Y, Asakura K, Yoshida Y, Watanabe SI, Asano N, Kawai A, Ohno M, Narita Y, Ishikawa M, Kato T, Fujimoto H, Niida S, Sakamoto H, Takizawa S, Akiba T, Okanohara D, Shiraishi K, Kohno T, Takeshita F, Nakagama H, Ota N, Ochiya T. Prediction of tissue-of-origin of early stage cancers using serum miRNomes. JNCI cancer spectrum, 7:pkac080, 2023
5. Udagawa H, Takahashi S, Hirao M, Tahara M, Iwasa S, Sato Y, Hamakawa T, Shitara K, Horinouchi H, Chin K, Masuda N, Suzuki T, Okumura S, Takase T, Nagai R, Yonemori K. Liposomal eribulin for advanced adenoid cystic carcinoma, gastric cancer, esophageal cancer, and small cell lung cancer. Cancer medicine, 12:1269-1278, 2023
6. Iwasa S, Koyama T, Nishino M, Kondo S, Sudo K, Yonemori K, Yoshida T, Tamura K, Shimizu T, Fujiwara Y, Kitano S, Shimomura A, Sato J, Yokoyama F, Iida H, Kondo M, Yamamoto N. First-in-human study of ONO-4578, an antagonist of prostaglandin E(2) receptor 4, alone and with nivolumab in solid tumors. Cancer science, 114:211-220, 2023
7. Takamizawa S, Katsuya Y, Chen YN, Mizuno T, Koyama T, Sudo K, Yoshida T, Kondo S, Iwasa S, Yonemori K, Shimizu T, Yamamoto N, Suzuki S. Ocular toxicity of investigational anti-cancer drugs in early phase clinical trials. Investigational new drugs, 41:173-181, 2023
8. Takamori H, Yamasaki T, Kitadai R, Minamishima YA, Nakamura E. Development of drugs targeting hypoxia-inducible factor against tumor cells with VHL mutation: Story of 127 years. Cancer science, 114:1208-1217, 2023
9. Kojima Y, Sudo K, Yoshida H, Yazaki S, Tokura M, Mizoguchi C, Okuma HS, Kita S, Yamamoto K, Nishikawa T, Noguchi E, Shimoi T, Tanase Y, Uno M, Ishikawa M, Kato T, Koyama K, Kobayashi M, Kakegawa T, Fujiwara Y, Yonemori K. Changes in HER3 expression profiles between primary and recurrent gynecological cancers. Cancer cell international, 23:18, 2023
10. Saito Y, Shimoi T, Iwata S, Maejima A, Abe K, Udagawa R, Yonemori K, Furukawa T, Wakao F. Impact of relative dose intensity of trabectedin with pegfilgrastim support: a single-centre retrospective study. Journal of chemotherapy (Florence, Italy), 1-8, 2023
11. Ishihara S, Ogura K, Maejima A, Shimoi T, Sudo K, Kojima Y, Fukushima S, Osaki S, Kobayashi E, Iwata S, Matsui Y, Yonemori K, Kawai A. Predictive value of peripheral blood markers in soft tissue sarcoma patients treated with eribulin. Japanese journal of clinical oncology, 53:494-500, 2023
12. Makise N, Shimoi T, Sunami K, Aoyagi Y, Kobayashi H, Tanaka S, Kawai A, Yonemori K, Ushiku T, Yoshida A. Loss of H3K27 trimethylation in a distinct group of de-differentiated chordoma of the skull base. Histopathology, 82:420-430, 2023
13. Schram AM, Colombo N, Arrowsmith E, Narayan V, Yonemori K, Scambia G, Zelnak A, Bauer TM, Jin N, Ulahannan SV, Colleoni M, Aftimos P, Donoghue MTA, Rosen E, Rudneva VA, Telli ML, Domchek SM, Galsky MD, Hoyle M, Chappey C, Stewart R, Blake-Haskins JA, Yap TA. Avelumab Plus Talazoparib in Patients With BRCA1/2- or ATM-Altered Advanced Solid Tumors: Results From JAVELIN BRCA/ATM, an Open-Label, Multicenter, Phase 2b, Tumor-Agnostic Trial. JAMA oncology, 9:29-39, 2023
14. Okuma HS, Yoshida H, Kobayashi Y, Arakaki M, Mizoguchi C, Inagaki L, Voon PJ, Malik Bin Ismail A, Fen Soo Hoo H, Yusak S, Severino B Imasa M, Nguyen Huy T, Thai Anh T, Kohsaka S, Mano H, Yonemori K, Nakamura K, Yatabe Y. Molecular pathology quality control in Southeast Asia: Results of a multiregional quality assurance study from MASTER KEY Asia. Cancer science, 114:2664-2673, 2023
15. Yagishita S, Nishikawa T, Yoshida H, Shintani D, Sato S, Miwa M, Suzuki M, Yasuda M, Ogitani Y, Jikoh T, Yonemori K, Hasegawa K, Hamada A. Co-clinical study of [fam-] trastuzumab deruxtecan (DS8201a) in patient-derived xenograft models of uterine carcinosarcoma and its association with clinical efficacy. Clinical cancer research, 29:2239-2249, 2023
16. Nishikawa T, Hasegawa K, Matsumoto K, Mori M, Hirashima Y, Takehara K, Ariyoshi K, Kato T, Yagishita S, Hamada A, Kawasaki M, Kawashima S, Tomatsuri S, Nagasaka Y, Yoshida H, Machida R, Hirakawa A, Nakamura K, Yonemori K. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. Journal of clinical oncology, 41:2789-2799, 2023
17. Sanomachi T, Okuma HS, Kitadai R, Kawachi A, Yazaki S, Tokura M, Arakaki M, Saito A, Kita S, Yamamoto K, Maejima A, Kojima Y, Nishikawa T, Sudo K, Shimoi T, Noguchi E, Fujiwara Y, Sugino H, Shiino S, Suto A, Yoshida M, Yonemori K. Low HER2 expression is a predictor of poor prognosis in stage I triple-negative breast cancer. Frontiers in oncology, 13:1157789, 2023
18. Shimomura A, Yoshida M, Kubo T, Yamashita S, Noguchi E, Nagayama A, Hanamura T, Okazaki M, Mukohara T, Tsuruga A, Tanaka K, Kawamura Y, Higuchi T, Takahashi Y, Kurozumi S, Hayashida T, Ichikawa H, Ushijima T, Suto A. Clinicopathological features, genetic alterations, and BRCA1 promoter methylation in Japanese male patients with breast cancer. Breast cancer research and treatment, 197:593-602, 2023
19. Sanomachi T, Sumiyoshi Okuma H, Yonemori K. COVID arm that appeared in the contralateral upper extremity after mRNA-1273 booster inoculation. International cancer conference journal, 12:216-219, 2023
20. Iwata H, Nakamura R, Masuda N, Yamashita T, Yamamoto Y, Kobayashi K, Tsurutani J, Iwasa T, Yonemori K, Tamura K, Aruga T, Tokunaga E, Kaneko K, Lee MJ, Yuno A, Kawabata A, Seike T, Kaneda A, Nishimura Y, Trepel JB, Saji S. Efficacy and exploratory biomarker analysis of entinostat plus exemestane in advanced or recurrent breast cancer: phase II randomized controlled trial. Japanese journal of clinical oncology, 53:4-15, 2023
21. Imoto S, Wang K, Bi XW, Liu G, Im YH, Im SA, Sim SH, Ueno T, Futamura M, Toi M, Fujiwara Y, Ahn SG, Lee JE, Park YH, Takao S, Oba MS, Kitagawa Y, Nishiyama M. Survival advantage of locoregional and systemic therapy in oligometastatic breast cancer: an international retrospective cohort study (OLIGO-BC1). Breast cancer (Tokyo, Japan), 30:412-423, 2023
22. Shitara K, Hirao M, Iwasa S, Oshima T, Komatsu Y, Kawazoe A, Sato Y, Hamakawa T, Yonemori K, Machida N, Yuki S, Suzuki T, Okumura S, Takase T, Semba T, Zimmermann B, Teng A, Yamaguchi K. Phase I Study of the Liposomal Formulation of Eribulin (E7389-LF): Results from the Advanced Gastric Cancer Expansion Cohort. Clinical cancer research, 29:1460-1467, 2023
23. Sase K, Mukai M, Fujiwara Y. Clinical Practice Guidelines in Cardio-Oncology: A Sea of Opportunity. JACC. CardioOncology, 5:145-148, 2023
24. Fujii H, Yamada Y, Yamamura K, Ishida Y, Tsujimura M, Matsumoto K, Tanaka S, Date H, Nishikawa T, Yoshida Y, Kashima J, Yatabe Y, Ogawa S, Marx A, Ulbright TM, Haga H. A case of vasculogenic mesenchymal tumor in the mediastinum: whole-exome sequencing reveals origin from pre-existing germ cell tumor. Virchows Archiv, 482:923-927, 2023
25. Tamura K, Mukohara T, Yonemori K, Kawabata Y, Nicolas X, Tanaka T, Iwata H. Phase 1 study of oral selective estrogen receptor degrader (SERD) amcenestrant (SAR439859), in Japanese women with ER-positive and HER2-negative advanced breast cancer (AMEERA-2). Breast cancer (Tokyo, Japan), 30:506-517, 2023
26. Chiba Y, Sudo K, Kojima Y, Okuma H, Kohsaka S, Machida R, Ichimura M, Anjo K, Kurishita K, Okita N, Nakamura K, Kinoshita I, Takahashi M, Matsubara J, Kusaba H, Yonemori K, Takahashi M. A multicenter investigator-initiated Phase 2 trial of E7090 in patients with advanced or recurrent solid tumor with fibroblast growth factor receptor (FGFR) gene alteration: FORTUNE trial. BMC cancer, 22:869, 2022
27. Omura T, Takahashi M, Ohno M, Miyakita Y, Yanagisawa S, Tamura Y, Kikuchi M, Kawauchi D, Nakano T, Hosoya T, Igaki H, Satomi K, Yoshida A, Sunami K, Hirata M, Shimoi T, Sudo K, Okuma HS, Yonemori K, Suzuki H, Ichimura K, Narita Y. Clinical Application of Comprehensive Genomic Profiling Tests for Diffuse Gliomas. Cancers, 14:2454, 2022
28. Koyama T, Shimizu T, Sato J, Katsuya Y, Iwasa S, Kondo S, Yoshida T, Sudo K, Nishino M, Takiguchi Y, Yonemori K, Yamamoto N. Practical consideration for successful sequential tumor biopsies in first-in-human trials. Investigational new drugs, 40:841-849, 2022
29. Kitadai R, Okuma Y. Treatment Strategies for Non-Small Cell Lung Cancer Harboring Common and Uncommon EGFR Mutations: Drug Sensitivity Based on Exon Classification, and Structure-Function Analysis. Cancers, 14:2519, 2022
30. Ida H, Koyama T, Mizuno T, Sunami K, Kubo T, Sudo K, Tao K, Hirata M, Yonemori K, Kato K, Okusaka T, Ohe Y, Matsui Y, Yamazaki N, Ogawa C, Kawai A, Narita Y, Esaki M, Yamamoto N. Clinical utility of comprehensive genomic profiling tests for advanced or metastatic solid tumor in clinical practice. Cancer science, 113:4300-4310, 2022
31. Yazaki S, Kojima Y, Yoshida H, Takamizawa S, Kitadai R, Nishikawa T, Shimoi T, Sudo K, Saito A, Okuma HS, Tanioka M, Noguchi E, Uno M, Ishikawa M, Kato T, Fujiwara Y, Ohe Y, Yonemori K. High expression of folate receptor alpha is associated with poor prognosis in patients with cervical cancer. Journal of gynecologic oncology, 33:e82, 2022
32. Udagawa C, Kuah S, Shimoi T, Kato K, Yoshida T, Nakano MH, Shimo A, Kojima Y, Yoshie R, Tsugawa K, Mushiroda T, Tan EY, Zembutsu H. Replication Study for the Association of Five SNPs Identified by GWAS and Trastuzumab-Induced Cardiotoxicity in Japanese and Singaporean Cohorts. Biological & pharmaceutical bulletin, 45:1198-1202, 2022
33. Kaku S, Horinouchi H, Watanabe H, Yonemori K, Okusaka T, Boku N, Yamazaki N, Kawai A, Ohe Y, Kusumoto M. Correction to: Incidence and prognostic factors in severe drug-induced interstitial lung disease caused by antineoplastic drug therapy in the real world. Journal of cancer research and clinical oncology, 148:1747, 2022
34. Matsui Y, Matsuda A, Maejima A, Shinoda Y, Nakamura E, Komiyama M, Fujimoto H. The clinical significance of perioperative inflammatory index as a prognostic factor for patients with retroperitoneal soft tissue sarcoma. International journal of clinical oncology, 27:1093-1100, 2022
35. Natsume T, Yoshida H, Nishikawa T, Kikkawa N, Naka T, Kobayashi-Kato M, Tanase Y, Uno M, Ishikawa M, Kato T. Uterine Leiomyosarcoma Masquerading as a Malignant Perivascular Epithelioid Cell Tumor: A Diagnostic Challenge. International journal of surgical pathology, 10668969221133348, 2022
36. Takamizawa S, Yazaki S, Kojima Y, Yoshida H, Kitadai R, Nishikawa T, Shimoi T, Sudo K, Okuma HS, Tanioka M, Noguchi E, Uno M, Ishikawa M, Kato T, Fujiwara Y, Yonemori K. High mesothelin expression is correlated with non-squamous cell histology and poor survival in cervical cancer: a retrospective study. BMC cancer, 22:1215, 2022
37. Kojima Y, Shimoi T, Seo T, Yazaki S, Okuya T, Ohtake Y, Okuma HS, Shimomura A, Nishikawa T, Tanioka M, Sudo K, Noguchi E, Tamura K, Yoshida A, Iwata S, Kobayashi E, Kawai A, Fujiwara Y, Yonemori K. Poor Treatment Outcomes with Second-Line Chemotherapy in Advanced Synovial Sarcoma. Oncology, 100:370-375, 2022
38. Takagi M, Ogawa C, Iehara T, Aoki-Nogami Y, Ishibashi E, Imai M, Kimura T, Nagata M, Yasuhara M, Masutani M, Yoshimura K, Tomizawa D, Ogawa A, Yonemori K, Morishita A, Miyamoto S, Takita J, Kihara T, Nobori K, Hasebe K, Miya F, Ikeda S, Shioda Y, Matsumoto K, Fujimura J, Mizutani S, Morio T, Hosoi H, Koike R. First phase 1 clinical study of olaparib in pediatric patients with refractory solid tumors. Cancer, 128:2949-2957, 2022
39. Yonemori K, Kuboki Y, Hasegawa K, Iwata T, Kato H, Takehara K, Hirashima Y, Kato H, Passey C, Buchbjerg JK, Harris JR, Andreassen CM, Nicacio L, Soumaoro I, Fujiwara K. Tisotumab vedotin in Japanese patients with recurrent/metastatic cervical cancer: Results from the innovaTV 206 study. Cancer science, 113:2788-2797, 2022
40. Hamamoto R, Koyama T, Kouno N, Yasuda T, Yui S, Sudo K, Hirata M, Sunami K, Kubo T, Takasawa K, Takahashi S, Machino H, Kobayashi K, Asada K, Komatsu M, Kaneko S, Yatabe Y, Yamamoto N. Introducing AI to the molecular tumor board: one direction toward the establishment of precision medicine using large-scale cancer clinical and biological information. Experimental hematology & oncology, 11:82, 2022
41. Yoshimoto D, Taguchi A, Tanikawa M, Sone K, Shimoi T, Tsuruga T, Oda K, Osuga Y. Recurrent cervical cancer with PD-L1 amplification treated with nivolumab: A case enrolled in the BELIEVE trial. The journal of obstetrics and gynaecology research, 48:2010-2014, 2022
42. Takamizawa S, Shimoi T, Yoshida M, Tokura M, Yazaki S, Mizoguchi C, Saito A, Kita S, Yamamoto K, Kojima Y, Sumiyoshi-Okuma H, Nishikawa T, Noguchi E, Sudo K, Yonemori K. Diagnostic value of tumor markers in identifying favorable or unfavorable subsets in patients with cancer of unknown primary: a retrospective study. BMC cancer, 22:412, 2022
43. Kojima N, Arai Y, Satomi K, Kubo T, Matsushita Y, Mori T, Matsushita H, Ushijima T, Yatabe Y, Shibata T, Yonemori K, Ichimura K, Ichikawa H, Kawai A, Yoshida A. Co-expression of ERG and CD31 in a subset of CIC-rearranged sarcoma: a potential diagnostic pitfall. Modern pathology, 35:1439-1448, 2022
44. Kawahara T, Kawai K, Kojima T, Nagumo Y, Sakka S, Kandori S, Negoro H, Mathis BJ, Maruo K, Miura K, Sakamoto N, Shinohara N, Yamashita S, Yonemori K, Kishida T, Ukimura O, Nishimura K, Kobayashi Y, Nishiyama H. Phase II trial of nivolumab monotherapy and biomarker screening in patients with chemo-refractory germ cell tumors. International journal of urology, 29:741-747, 2022
45. Yonemori K, Yunokawa M, Ushijima K, Sakata J, Shikama A, Minobe S, Usami T, Enomoto T, Takehara K, Hasegawa K, Yamagami W, Yamamoto K, Han S, Dutta L, Orlowski R, Miura T, Makker V, Fujiwara K. Lenvatinib plus pembrolizumab in Japanese patients with endometrial cancer: Results from Study 309/KEYNOTE-775. Cancer science, 113:3489-3497, 2022
46. Saito A, Tanioka M, Hirata M, Watanabe T, Odaka Y, Shimoi T, Sudo K, Noguchi E, Ishikawa M, Yonemori K. Case report: Response to platinum agents and poly (adenosine diphosphate-ribose) polymerase inhibitor in a patient with BRCA1 c.5096G>A (R1699Q) intermediate-risk variant. Cancer treatment and research communications, 32:100587, 2022
47. Nishio S, Yonemori K, Usami T, Minobe S, Yunokawa M, Iwata T, Okamoto A, Aoki Y, Itamochi H, Takekuma M, Harano K, Yamamoto K, Maruko T, Ugai H, Tekin C, Colombo N, Fujiwara K, Hasegawa K, Ushijima K. Pembrolizumab plus chemotherapy in Japanese patients with persistent, recurrent or metastatic cervical cancer: Results from KEYNOTE-826. Cancer science, 113:3877-3887, 2022
48. Farley JH, Brady WE, O’Malley D, Fujiwara K, Yonemori K, Bonebrake A, Secord AA, Stephan JM, Walker JL, Nam JH, Birrer MJ, Gershenson DM. A phase II evaluation of temsirolimus with carboplatin and paclitaxel followed by temsirolimus consolidation in clear cell ovarian cancer: An NRG oncology trial. Gynecologic oncology, 167:423-428, 2022
49. Ebata T, Yamashita S, Takeshima H, Yoshida H, Kawata Y, Kino N, Yasugi T, Terao Y, Yonemori K, Kato T, Ushijima T. DNA methylation of the immediate upstream region of BRCA1 major transcription start sites is an independent favorable prognostic factor in patients with high-grade serous ovarian cancer. Gynecologic oncology, 167:513-518, 2022
50. Tashiro R, Kawazoe H, Mamishin K, Seto K, Udagawa R, Saito Y, Hashimoto H, Shimoi T, Yonemori K, Yonemura M, Terakado H, Kawasaki T, Furukawa T, Nakamura T. Patient-associated risk factors for severe anemia in patients with advanced ovarian or breast cancer receiving olaparib monotherapy: A multicenter retrospective study. Frontiers in oncology, 12:898150, 2022
51. Masuda N, Ono M, Mukohara T, Yasojima H, Shimoi T, Kobayashi K, Harano K, Mizutani M, Tanioka M, Takahashi S, Kogawa T, Suzuki T, Okumura S, Takase T, Nagai R, Semba T, Zhao ZM, Ren M, Yonemori K. Phase 1 study of the liposomal formulation of eribulin (E7389-LF): Results from the breast cancer expansion cohort. European journal of cancer (Oxford, England : 1990), 168:108-118, 2022
52. Uneno Y, Iwai M, Morikawa N, Tagami K, Matsumoto Y, Nozato J, Kessoku T, Shimoi T, Yoshida M, Miyoshi A, Sugiyama I, Mantani K, Itagaki M, Yamagishi A, Morita T, Inoue A, Muto M. Development of a national health policy logic model to accelerate the integration of oncology and palliative care: a nationwide Delphi survey in Japan. International journal of clinical oncology, 27:1529-1542, 2022
53. Kotani H, Masuda N, Yamashita T, Naito Y, Taira T, Inoue K, Takahashi M, Yonemori K, Toyoizumi S, Mori Y, Nagasawa T, Hori N, Iwata H. Efficacy and safety of talazoparib in Japanese patients with germline BRCA-mutated locally advanced or metastatic breast cancer: results of the phase 1 dose-expansion study. Breast cancer (Tokyo, Japan), 29:1088-1098, 2022
