Annual Report 2022
Department of Gastrointestinal Medical Oncology
Ken Kato, Atsuo Takashima, Hirokazu Shoji, Hidekazu Hirano, Toshiharu Hirose, Natsuko Okita, Shun Yamamoto Yoshitaka Honma, Yuri Yoshinami, Nozomu Ogura, Takahito Awatsu, Kunihito Matuguma
Introduction
The Department of Gastrointestinal Medical Oncology specializes in the medical treatment of malignant tumors originating in the digestive tract, including gastric cancer, esophagogastric junction cancer, colorectal cancer, and rare cancers of the digestive tract such as anal canal cancer, gastrointestinal stromal tumors (GISTs), and small intestine cancer. They collaborate with the Departments of Gastrointestinal Surgery, Colorectal Surgery, and Gastrointestinal Endoscopy to provide comprehensive treatment.
The Team and What We Do
In the outpatient setting, the staff takes the lead, with each member responsible for clinic sessions for 2 to 3 days per week. In the inpatient setting, a team consisting of full-time physicians, cancer specialist trainees, and residents collaboratively provide patient care. Treatment strategies are determined through departmental conferences held in the morning and evening, as well as weekly conferences specifically for gastric cancer and colorectal cancer cases.
In the fiscal year 2022, there were a total of 375 new patients, excluding those in the Head and Neck/Esophagus Department. The number of inpatients reached 835, including 250 with gastric cancer, 38 with esophagogastric junction cancer, 377 with colorectal cancer, 44 with anal canal cancer, 22 with GISTs, 25 with small intestine cancer, and 79 with other conditions.
Research Activities
With the aim of establishing better treatment methods, our department actively engages in clinical research. Our staff members play a central role in conceiving new trials for the Japan Clinical Oncology Group (JCOG) and the West Japan Oncology Group (WJOG), actively participating in developing new standard treatments. As an institution, we proactively enroll patients in these trials, contributing to the establishment of novel treatment standards.
Furthermore, we participate in domestic and international clinical trials ranging from Phase I to Phase III, not only for late-stage treatment development but also for developing new drugs, starting from the planning stage. Additionally, we conduct physician-led trials for second-line treatment of gastric cancer. We actively collaborate in translational research (TR) with research institutes and other basic research facilities and propose new physician-led trials to pharmaceutical companies.
We published 24 papers, of which 22 were in English-language journals and 2 were English-language reviews. Among these, our department was the lead or responsible author in 8 papers.
Clinical Trials
In the fiscal year 2022, a total of 146 patients were enrolled in clinical trials, as shown in Table 1. We presented 32 papers at conferences, with 14 of them being international conferences. (Table 1).
Table 1. Clinical trials and the number of registered patients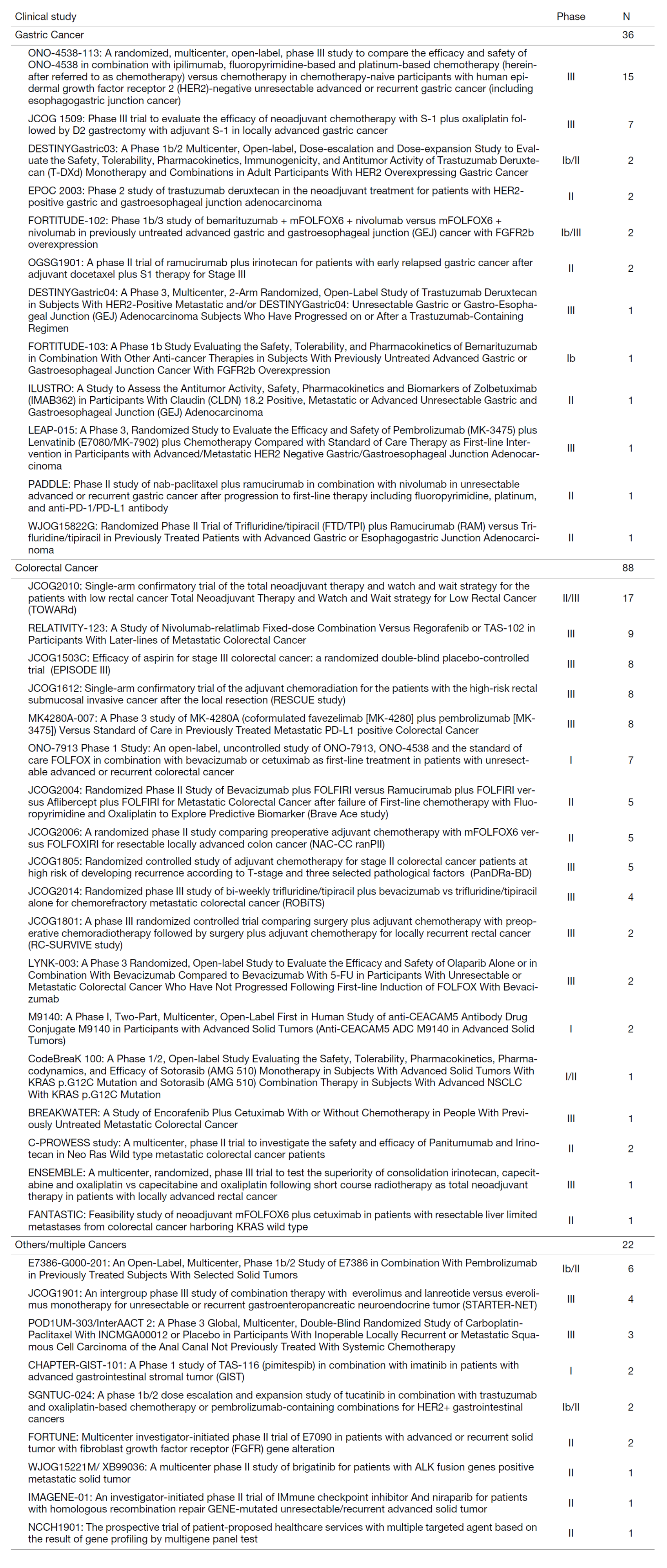

Education
In patient care, full-time physicians collaborate with cancer specialist trainees/residents to form teams and provide guidance through their interactions with assigned patients. Additionally, cancer specialist trainees/residents are assigned research topics and receive active guidance in the developing, managing, presenting at conferences, and writing papers related to clinical research. In the fiscal year 2022, cancer specialist trainees/residents in our department authored a total of 7 English-language papers as lead authors.
Future Prospects
We are committed to giving our all in patient care, research, and education to further improve the treatment outcomes for gastrointestinal cancers. In patient care, we adhere to standard treatments as a foundation, but we also strive to be innovative and continuously improve by addressing unmet patient needs, aiming for "always better." We are also keen to foster collaboration with local hospitals.
In the realm of research, we continue to lead in conducting clinical trials and multicenter collaborative clinical studies using our specialized expertise and effectively use translational research (TR) conducted in collaboration with research institutes. Our goal is to develop treatment approaches that have the potential to become the next standard of care. We actively engage in web conferences with industry partners and international researchers to bring a broader perspective to our development efforts.
In terms of education, we aim not only to educate residents about cancer pharmacotherapy but also to nurture young oncologists with a strong research mindset. We want to develop the next generation of researchers who are not only highly specialized but also have the ability to think critically and creatively.
List of papers published in 2022
Journal
1. Omura G, Honma Y, Matsumoto Y, Shinozaki T, Itoyama M, Eguchi K, Sakai T, Yokoyama K, Watanabe T, Ohara A, Kato K, Yoshimoto S. Transnasal photoimmunotherapy with cetuximab sarotalocan sodium: Outcomes on the local recurrence of nasopharyngeal squamous cell carcinoma. Auris, nasus, larynx, 50:641-645, 2023
2. Kadono T, Yamamoto S, Hirose T, Ikeda G, Ohara A, Itoyama M, Yokoyama K, Honma Y, Hashimoto T, Sekine S, Ishiyama K, Oguma J, Daiko H, Kato K. Safety and short-term efficacy of preoperative FOLFOX therapy in patients with resectable esophageal squamous cell carcinoma who are ineligible for cisplatin. Esophagus, 20:109-115, 2023
3. Kato K, Doki Y, Ogata T, Motoyama S, Kawakami H, Ueno M, Kojima T, Shirakawa Y, Okada M, Ishihara R, Kubota Y, Amaya-Chanaga C, Chen T, Matsumura Y, Kitagawa Y. First-line nivolumab plus ipilimumab or chemotherapy versus chemotherapy alone in advanced esophageal squamous cell carcinoma: a Japanese subgroup analysis of open-label, phase 3 trial (CheckMate 648/ONO-4538-50). Esophagus, 20:291-301, 2023
4. Mitani S, Kato K, Daiko H, Ito Y, Nozaki I, Kojima T, Yano M, Nakagawa S, Ueno M, Watanabe M, Tsunoda S, Abe T, Kadowaki S, Kadota T, Sasaki K, Machida R, Kitagawa Y. Second primary malignancies in patients with clinical T1bN0 esophageal squamous cell carcinoma after definitive therapies: supplementary analysis of the JCOG trial: JCOG0502. Journal of gastroenterology, 57:455-463, 2022
5. Takeuchi H, Ito Y, Machida R, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Okuno T, Hironaka S, Nozaki I, Ura T, Chin K, Kojima T, Seki S, Sakanaka K, Fukuda H, Kitagawa Y. A Single-Arm Confirmatory Study of Definitive Chemoradiation Therapy Including Salvage Treatment for Clinical Stage II/III Esophageal Squamous Cell Carcinoma (JCOG0909 Study). International journal of radiation oncology, biology, physics, 114:454-462, 2022
6. Takemura C, Kashima J, Hashimoto T, Ichikawa H, Honma Y, Goto Y, Watanabe SI, Yatabe Y. A mimic of lung adenocarcinoma: a case report of histological conversion of metastatic thyroid papillary carcinoma. Histopathology, 80:1004-1007, 2022
7. Ida H, Koyama T, Mizuno T, Sunami K, Kubo T, Sudo K, Tao K, Hirata M, Yonemori K, Kato K, Okusaka T, Ohe Y, Matsui Y, Yamazaki N, Ogawa C, Kawai A, Narita Y, Esaki M, Yamamoto N. Clinical utility of comprehensive genomic profiling tests for advanced or metastatic solid tumor in clinical practice. Cancer science, 113:4300-4310, 2022
8. Oguma J, Ishiyama K, Kurita D, Kanematsu K, Fujii Y, Kubo K, Yamamoto S, Honma Y, Kato K, Daiko H. Novel pathological staging for patients with locally advanced esophageal squamous cell carcinoma undergoing neoadjuvant chemotherapy followed by surgery. Esophagus, 19:214-223, 2022
9. Tsukamoto S, Honma Y, Shoji H, Hirano H, Inoue M, Takamizawa Y, Moritani K, Imaizumi J, Kanemitsu Y. Clinical outcomes of surgical and imatinib treatment for rectal gastrointestinal stromal tumours: retrospective cohort study. BJS open, 6:zrac067, 2022
10. Shibayama T, Shimoi T, Mori T, Noguchi E, Honma Y, Hijioka S, Yoshida M, Ogawa C, Yonemori K, Yatabe Y, Yoshida A. Cytokeratin-positive Malignant Tumor in the Abdomen With EWSR1/FUS-CREB Fusion: A Clinicopathologic Study of 8 Cases. The American journal of surgical pathology, 46:134-146, 2022
11. Kang YK, Morita S, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Sameshima H, Chen LT, Boku N. Exploration of predictors of benefit from nivolumab monotherapy for patients with pretreated advanced gastric and gastroesophageal junction cancer: post hoc subanalysis from the ATTRACTION-2 study. Gastric cancer, 25:207-217, 2022
12. Sakai M, Kitagawa Y, Saeki H, Miyazaki T, Yamaji T, Nemoto K, Oyama T, Muto M, Takeuchi H, Toh Y, Matsubara H, Mano M, Kono K, Kato K, Yoshida M, Kawakubo H, Booka E, Yamatsuji T, Kato H, Ito Y, Ishikawa H, Ishihara R, Tsushima T, Kawachi H, Oyama T, Kojima T, Kuribayashi S, Makino T, Matsuda S, Sohda M, Kubo Y, Doki Y. Fruit and vegetable consumption and risk of esophageal cancer in the Asian region: a systematic review and meta-analysis. Esophagus, 19:27-38, 2022
13. Muro K, Kojima T, Moriwaki T, Kato K, Nagashima F, Kawakami H, Ishihara R, Ogata T, Satoh T, Iwakami K, Han S, Yatsuzuka N, Takami T, Bhagia P, Doi T. Second-line pembrolizumab versus chemotherapy in Japanese patients with advanced esophageal cancer: subgroup analysis from KEYNOTE-181. Esophagus, 19:137-145, 2022
14. Kubo Y, Kitagawa Y, Miyazaki T, Sohda M, Yamaji T, Sakai M, Saeki H, Nemoto K, Oyama T, Muto M, Takeuchi H, Toh Y, Matsubara H, Mano M, Kono K, Kato K, Yoshida M, Kawakubo H, Booka E, Yamatsuji T, Kato H, Ito Y, Ishikawa H, Ishihara R, Tsushima T, Kawachi H, Oyama T, Kojima T, Kuribayashi S, Makino T, Matsuda S, Doki Y. The potential for reducing alcohol consumption to prevent esophageal cancer morbidity in Asian heavy drinkers: a systematic review and meta-analysis. Esophagus, 19:39-46, 2022
15. Oshima K, Kato K, Ito Y, Daiko H, Nozaki I, Nakagawa S, Shibuya Y, Kojima T, Toh Y, Okada M, Hironaka S, Akiyama Y, Komatsu Y, Maejima K, Nakagawa H, Onuki R, Nagai M, Kato M, Kanato K, Kuchiba A, Nakamura K, Kitagawa Y. Prognostic biomarker study in patients with clinical stage I esophageal squamous cell carcinoma: JCOG0502-A1. Cancer science, 113:1018-1027, 2022
16. Kashihara T, Ishiki H, Kato K. Definitive Chemoradiotherapy for Older Patients With Esophageal Cancer. JAMA oncology, 8:304-305, 2022
17. Yoshikawa AK, Yamaguchi K, Muro K, Takashima A, Ichimura T, Sakai D, Kadowaki S, Chin K, Kudo T, Mitani S, Kitano S, Thai D, Zavodovskaya M, Liu J, Boku N, Satoh T. Safety and tolerability of andecaliximab as monotherapy and in combination with an anti-PD-1 antibody in Japanese patients with gastric or gastroesophageal junction adenocarcinoma: a phase 1b study. Journal for immunotherapy of cancer, 10:e003518, 2022
18. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. The Lancet. Oncology, 23:234-247, 2022
19. Mori Y, Kikuchi O, Horimatsu T, Hara H, Hironaka S, Kojima T, Kato K, Tsushima T, Ishihara R, Mukai K, Uozumi R, Tada H, Kasai H, Kawaguchi A, Muto M. Multicenter phase II study of trifluridine/tipiracil for esophageal squamous carcinoma refractory/intolerant to 5-fluorouracil, platinum compounds, and taxanes: the ECTAS study. Esophagus, 19:444-451, 2022
20. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. The New England journal of medicine, 386:449-462, 2022
21. Kikuchi K, Yamazaki N, Nozawa K, Fukuda H, Shibata T, Machida R, Hamaguchi T, Takashima A, Shoji H, Boku N, Takatsuka S, Takenouchi T, Nishina T, Yoshikawa S, Takahashi M, Hasegawa A, Kawazoe A, Masuishi T, Mizutani H, Kiyohara Y. Topical corticosteroid therapy for facial acneiform eruption due to EGFR inhibitors in metastatic colorectal cancer patients: a randomized controlled trial comparing starting with a very strong or a weak topical corticosteroid (FAEISS study, NCCH1512, colorectal part). Supportive care in cancer, 30:4497-4504, 2022
22. Kato K, Kadota T, Abe S. Reply. Gastroenterology, 162:2130-2131, 2022
23. Nakamura Y, Okamoto W, Denda T, Nishina T, Komatsu Y, Yuki S, Yasui H, Esaki T, Sunakawa Y, Ueno M, Shinozaki E, Matsuhashi N, Ohta T, Kato K, Ohtsubo K, Bando H, Hara H, Satoh T, Yamazaki K, Yamamoto Y, Okano N, Terazawa T, Kato T, Oki E, Tsuji A, Horita Y, Hamamoto Y, Kawazoe A, Nakajima H, Nomura S, Mitani R, Yuasa M, Akagi K, Yoshino T. Clinical Validity of Plasma-Based Genotyping for Microsatellite Instability Assessment in Advanced GI Cancers: SCRUM-Japan GOZILA Substudy. JCO precision oncology, 6:e2100383, 2022
24. Suzuki K, Igata H, Abe M, Yamamoto Y. Multiple cancer type classification by small RNA expression profiles with plasma samples from multiple facilities. Cancer science, 113:2144-2166, 2022
25. Tsubokura M, Adegawa Y, Kojima M, Tanosaki R, Ohtake R, Kase Y, Iwashita N, Kasane M, Nakabayashi S, Takeuchi S, Kato K, Boku N, Kanemitsu Y, Okusaka T, Fujimoto H, Yonemori K, Ishiki H, Kawamura K, Satomi E, Matsushita H. Adverse effects of cell-free and concentrated ascites reinfusion therapy for malignant ascites: a single-institute experience. BMC cancer, 22:268, 2022
26. Okada M, Kato K, Cho BC, Takahashi M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Matsumura Y, Takazawa A, Kitagawa Y. Three-Year Follow-Up and Response-Survival Relationship of Nivolumab in Previously Treated Patients with Advanced Esophageal Squamous Cell Carcinoma (ATTRACTION-3). Clinical cancer research, 28:3277-3286, 2022
27. Hirano H, Abe Y, Nojima Y, Aoki M, Shoji H, Isoyama J, Honda K, Boku N, Mizuguchi K, Tomonaga T, Adachi J. Temporal dynamics from phosphoproteomics using endoscopic biopsy specimens provides new therapeutic targets in stage IV gastric cancer. Scientific reports, 12:4419, 2022
28. Inoue T, Ishihara R, Shibata T, Suzuki K, Kitagawa Y, Miyazaki T, Yamaji T, Nemoto K, Oyama T, Muto M, Takeuchi H, Toh Y, Matsubara H, Mano M, Kono K, Kato K, Yoshida M, Kawakubo H, Booka E, Yamatsuji T, Kato H, Ito Y, Ishikawa H, Tsushima T, Kawachi H, Oyama T, Kojima T, Kuribayashi S, Makino T, Matsuda S, Doki Y. Endoscopic imaging modalities for diagnosing the invasion depth of superficial esophageal squamous cell carcinoma: a systematic review. Esophagus, 19:375-383, 2022
29. Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, Yu X, Shu Y, Luo Q, Wang J, Chen Z, Niu Z, Zhang L, Yi T, Sun JM, Chen J, Yu G, Lin CY, Hara H, Bi Q, Satoh T, Pazo-Cid R, Arkenau HT, Borg C, Lordick F, Li L, Ding N, Tao A, Shi J, Van Cutsem E. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. Journal of clinical oncology, 40:3065-3076, 2022
30. Mizukami T, Takahashi M, Sunakawa Y, Yuki S, Kagawa Y, Takashima A, Kato K, Hara H, Denda T, Yamamoto Y, Shiozawa M, Oki E, Okamoto W, Yoshino T, Eguchi Nakajima T. Genomic Landscape of Primary Tumor Site and Clinical Outcome for Patients with Metastatic Colorectal Cancer Receiving Standard-of-Care Chemotherapy. Targeted oncology, 17:343-353, 2022
31. Kajiwara T, Nishina T, Nakasya A, Yamashita N, Yamashita R, Nakamura Y, Shiozawa M, Yuki S, Taniguchi H, Hara H, Ohta T, Esaki T, Shinozaki E, Takashima A, Moriwaki T, Denda T, Ohtsubo K, Sunakawa Y, Horita Y, Kawakami H, Kato T, Satoh T, Ando K, Mizutani T, Yasui H, Goto M, Okuyama H, Yamazaki K, Yoshino T, Hyodo I. NOTCH gene alterations in metastatic colorectal cancer in the Nationwide Cancer Genome Screening Project in Japan (SCRUM-Japan GI-SCREEN). Journal of cancer research and clinical oncology, 148:2841-2854, 2022
32. Itami J, Kobayashi K, Mori T, Honma Y, Kubo Y, Murakami N, Omura G, Okuma K, Inaba K, Takahashi K, Kashihara T, Shimizu Y, Takahashi A, Nakayama Y, Matsumoto F, Yoshimoto S, Igaki H. Non-Robustness of Ang’s Risk Classification in Human Papillomavirus-Related Oropharyngeal Squamous Cell Carcinoma in Japanese Patients. Cancers, 14:2442, 2022
33. Oshima K, Hirano H, Shoji H, Iwasa S, Okita N, Takashima A, Boku N. Influence of precedent drug on the subsequent therapy in the sequence of trifluridine/tipiracil with/out bevacizumab and regorafenib for unresectable or recurrent colorectal cancer. PloS one, 17:e0269115, 2022
34. Kojima T, Hara H, Tsuji A, Yasui H, Muro K, Satoh T, Ogata T, Ishihara R, Goto M, Baba H, Nishina T, Han S, Sakata T, Yatsuzuka N, Doi T, Kato K. First-line pembrolizumab + chemotherapy in Japanese patients with advanced/metastatic esophageal cancer from KEYNOTE-590. Esophagus, 19:683-692, 2022
35. Kurokawa Y, Honma Y, Sawaki A, Naito Y, Iwagami S, Komatsu Y, Takahashi T, Nishida T, Doi T. Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): a randomized, double-blind, placebo-controlled phase III trial. Annals of oncology, 33:959-967, 2022
36. Yamaguchi K, Minashi K, Sakai D, Nishina T, Omuro Y, Tsuda M, Iwagami S, Kawakami H, Esaki T, Sugimoto N, Oshima T, Kato K, Amagai K, Hosaka H, Komine K, Yasui H, Negoro Y, Ishido K, Tsushima T, Han S, Shiratori S, Takami T, Shitara K. Phase IIb study of pembrolizumab combined with S-1 + oxaliplatin or S-1 + cisplatin as first-line chemotherapy for gastric cancer. Cancer science, 113:2814-2827, 2022
37. Hashimoto T, Takayanagi D, Yonemaru J, Naka T, Nagashima K, Yatabe Y, Shida D, Hamamoto R, Kleeman SO, Leedham SJ, Maughan T, Takashima A, Shiraishi K, Sekine S. Clinicopathological and molecular characteristics of RSPO fusion-positive colorectal cancer. British journal of cancer, 127:1043-1050, 2022
38. Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, Toh Y, Doki Y, Naomoto Y, Nemoto K, Booka E, Matsubara H, Miyazaki T, Muto M, Yanagisawa A, Yoshida M. Correction to: Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1 and Part 2. Esophagus, 19:726, 2022
39. Ooki A, Satoh T, Muro K, Takashima A, Kadowaki S, Sakai D, Ichimura T, Mitani S, Kudo T, Chin K, Kitano S, Thai D, Zavodovskaya M, Liu J, Boku N, Yamaguchi K. A phase 1b study of andecaliximab in combination with S-1 plus platinum in Japanese patients with gastric adenocarcinoma. Scientific reports, 12:11007, 2022
40. Van Cutsem E, Kato K, Ajani J, Shen L, Xia T, Ding N, Zhan L, Barnes G, Kim SB. Tislelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma (RATIONALE 302): impact on health-related quality of life. ESMO open, 7:100517, 2022
41. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Lockhart AC, Arkenau HT, El-Hajbi F, Gupta M, Pfeiffer P, Bhagia P, Cao ZA, Lunceford J, Suryawanshi S, Ayers M, J Marton M, Kato K. T cell-inflamed gene expression profile and PD-L1 expression and pembrolizumab efficacy in advanced esophageal cancer. Future oncology (London, England), 18:2783-2790, 2022
42. Ito T, Takashima A, Yamazaki K, Yukami H, Uetake H, Tsuda M, Suto T, Moriwaki T, Sugimoto N, Ojima H, Takii Y, Yasui H, Esaki T, Tsuji A, Goto M, Saruta M, Otsu S, Shinozaki K, Fujiwara T, Tamura T, Baba E, Shiozawa M, Denda T, Ueno H, Nagashima K, Shimada Y. Primary tumor location as a predictor of survival in patients with RAS wild-type colorectal cancer who receive molecularly targeted drugs as first-line therapy: a multicenter real-world observational study by the Japanese Society for Cancer of the Colon and Rectum. International journal of clinical oncology, 27:1450-1458, 2022
43. Kojima T, Kato K, Hara H, Takahashi S, Muro K, Nishina T, Wakabayashi M, Nomura S, Sato A, Ohtsu A, Doi T. Phase II study of BKM120 in patients with advanced esophageal squamous cell carcinoma (EPOC1303). Esophagus, 19:702-710, 2022
44. Udagawa C, Kuah S, Shimoi T, Kato K, Yoshida T, Nakano MH, Shimo A, Kojima Y, Yoshie R, Tsugawa K, Mushiroda T, Tan EY, Zembutsu H. Replication Study for the Association of Five SNPs Identified by GWAS and Trastuzumab-Induced Cardiotoxicity in Japanese and Singaporean Cohorts. Biological & pharmaceutical bulletin, 45:1198-1202, 2022
45. Hino K, Nishina T, Kajiwara T, Bando H, Nakamura M, Kadowaki S, Minashi K, Yuki S, Ohta T, Hara H, Mizukami T, Moriwaki T, Ohtsubo K, Komoda M, Mitani S, Nagashima F, Kato K, Yamada T, Hasegawa H, Yamazaki K, Yoshino T, Hyodo I. Association of ERBB2 Copy Number and Gene Coalterations With Trastuzumab Efficacy and Resistance in Human Epidermal Growth Factor Receptor 2-Positive Esophagogastric and Gastric Cancer. JCO precision oncology, 6:e2200135, 2022
46. Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J. Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA oncology, 8:1447-1455, 2022
47. Shimizu Y, Murakami N, Mori T, Takahashi K, Kubo Y, Yoshimoto S, Honma Y, Nakamura S, Okamoto H, Iijima K, Takahashi A, Kaneda T, Kashihara T, Inaba K, Okuma K, Nakayama Y, Igaki H, Itami J. Clinical impact of p16 positivity in nasopharyngeal carcinoma. Laryngoscope investigative otolaryngology, 7:994-1001, 2022
48. Kawakita D, Nagao T, Takahashi H, Kano S, Honma Y, Hirai H, Saigusa N, Akazawa K, Tani K, Ojiri H, Tsukahara K, Ozawa H, Okami K, Kondo T, Togashi T, Fushimi C, Shimura T, Shimizu A, Okamoto I, Okada T, Imanishi Y, Watanabe Y, Otsuka K, Sakai A, Ebisumoto K, Sato Y, Yamazaki K, Ueki Y, Hanazawa T, Saito Y, Ando M, Matsuki T, Nakaguro M, Sato Y, Urano M, Utsumi Y, Kohsaka S, Saotome T, Tada Y. Survival benefit of HER2-targeted or androgen deprivation therapy in salivary duct carcinoma. Therapeutic advances in medical oncology, 14:17588359221119538, 2022
49. Shimozaki K, Hirata K, Sato T, Nakamura M, Kato K, Hirano H, Kumekawa Y, Hino K, Kawakami K, Kito Y, Matsumoto T, Kawakami T, Komoda M, Nagashima K, Sato Y, Yamazaki K, Hironaka S, Takaishi H, Hamamoto Y, Muro K. WJOG13219G: The Efficacy and Safety of FOLFOXIRI or Doublet plus Anti-VEGF Therapy in Previously Untreated BRAF(V600E) Mutant Metastatic Colorectal Cancer: A Multi-Institutional Registry-Based Study (BRACELET Study). Clinical colorectal cancer, 21:339-346, 2022
50. Miyata Y, Murakami N, Honma Y, Mori T, Yoshimoto S, Kashihara T, Takemori M, Nakayama Y, Itami J, Ogo E, Igaki H. Technical report: a high-dose-rate interstitial brachytherapy boost for residual sinonasal undifferentiated carcinoma. Journal of radiation research, 63:879-883, 2022
51. Matsuoka A, Fujimori M, Narikazu B, Takashima A, Okusaka T, Mori K, Akechi T, Shimazu T, Okizaki A, Miyaji T, Majima Y, Nagashima F, Uchitomi Y. Geriatric assessment and management with question prompt list using a web-based application for elderly patients with cancer (MAPLE) to communicate ageing-related concerns: J-SUPPORT 2101 study protocol for a multicentre, parallel group, randomised controlled trial. BMJ open, 12:e063445, 2022
52. Yamamoto S, Sakakibara N, Hirano H, Morizane C, Honma Y, Hijioka S, Okusaka T, Higashi T, Kawai A. The real-world selection of first-line systemic therapy regimen for metastatic gastroenteropancreatic neuroendocrine neoplasm in Japan. Scientific reports, 12:17601, 2022
53. Saigusa N, Hirai H, Tada Y, Kawakita D, Nakaguro M, Tsukahara K, Kano S, Ozawa H, Kondo T, Okami K, Togashi T, Sato Y, Urano M, Kajiwara M, Shimura T, Fushimi C, Shimizu A, Okamoto I, Okada T, Suzuki T, Imanishi Y, Watanabe Y, Sakai A, Ebisumoto K, Sato Y, Honma Y, Yamazaki K, Ueki Y, Hanazawa T, Saito Y, Takahashi H, Ando M, Kohsaka S, Matsuki T, Nagao T. The Role of the EZH2 and H3K27me3 Expression as a Predictor of Clinical Outcomes in Salivary Duct Carcinoma Patients: A Large-Series Study With Emphasis on the Relevance to the Combined Androgen Blockade and HER2-Targeted Therapy. Frontiers in oncology, 11:779882, 2021
