Annual Report 2022
Department of Hepatobiliary and Pancreatic Oncology
Takuji Okusaka, Hideki Ueno, Chigusa Morizane, Susumu Hijioka, Shunsuke Kondo, Akihiro Ohba, Yoshikuni Nagashio, Yuta Maruki, Kotaro Takeshita, Tetsuro Takasaki
Introduction
The Department of Hepatobiliary and Pancreatic Oncology treats tumors originating from the liver, biliary system or pancreas, including hepatocellular carcinoma (HCC), biliary tract cancer and pancreatic cancer. We work closely with surgeons and radiologists who have special expertise in these areas as part of the multi-disciplinary care given at the National Cancer Center Hospital (NCCH). We also conduct clinical and translational research on hepatobiliary and pancreatic tumors and aim to develop new and more effective diagnostic methods and treatments.
The Team and What We Do
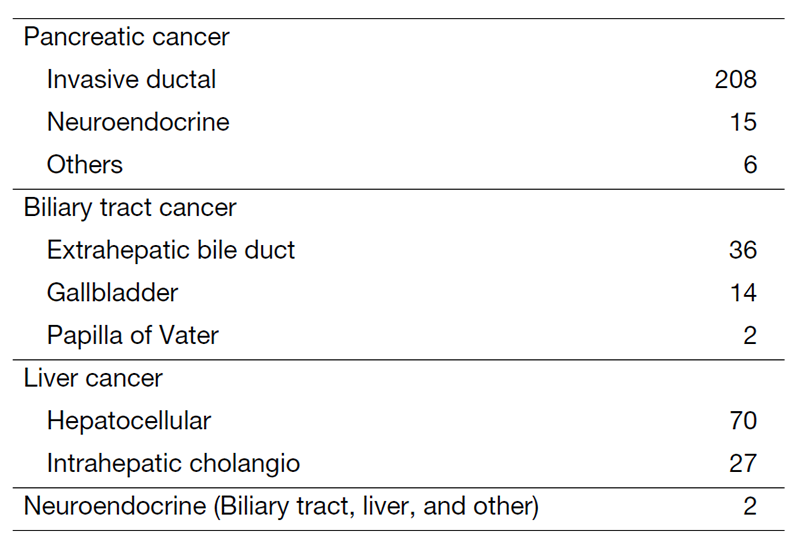
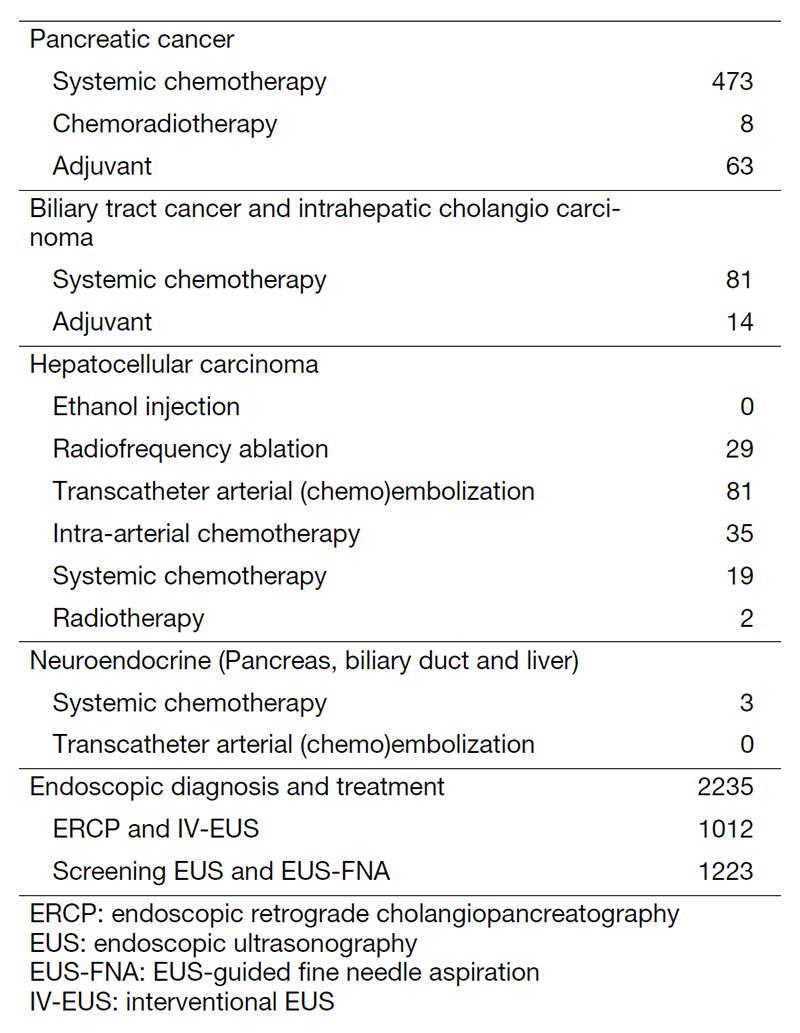
The department consists of eight staff oncologists and several residents. We have used percutaneous ablation therapy for most patients with three or fewer HCC nodules, all of which are smaller than 3 cm in diameter. We also perform transcatheter arterial chemoembolization (TACE), mainly with patients with multiple HCC nodules. Systemic or intra-arterial chemotherapeutic regimens are indicated in patients with advanced HCC for whom locoregional intervention and surgery are unsuitable or have been unsuccessful. In patients with unresectable pancreatic cancer or biliary tract cancer, chemotherapy is performed in clinical practice or as a clinical trial to develop new treatment. We have actively introduced endoscopic procedures for imaging diagnosis (endoscopic ultrasonography (EUS) and endoscopic retrograde cholangiopancreatography (ERCP)), tumor biopsy (EUS-guided tissue acquisition (EUS-TA)), and biliary drainage (including EUS-guided hepaticogastrostomy (EUS-HGS) and EUS-guided choledochoduodenostomy (EUS-CDS)) (Tables 1 and 2).
Research Activities
We published 65 papers as a first author in peer-reviewed journals in 2022.
Clinical Trials
Thirty clinical studies are ongoing, including four phase I studies, 11 phase II studies, and six phase III studies, such as adjuvant chemotherapy after resection versus resection alone for patients with resectable tumors, chemotherapy with a new regimen versus standard therapy for patients with advanced tumors, and several observation or registration trials. Our studies are supported by the National Cancer Center Research and Development Fund (2023-J-03), Project for Development of Innovative Research on Cancer Therapeutics (22ck0106492h0004, 22ck0106625h0003, 22ck0106766h0001) from the Japan Agency for Medical Research and Development.
Education
Our staff members are working closely with residents to support their skill development and knowledge expansion in both clinical and research fields. We are conducting conferences daily for clinical practice and weekly for research development. The residents in our department published 22 papers as a first author in peer-reviewed journals in 2022, and are performing 30 planning-stage or ongoing studies as a leading researcher, with assistance from staff members.
Future Prospects
Our department will continue to provide the best and latest diagnosis, treatment and supportive care, and develop more effective methods and techniques for all patients with hepatobiliary and pancreatic cancer in this country and globally. Among these, conducting clinical trials with novel promising agents for this disease is considered one of the most important tasks, and establishing cutting-edge endoscopic procedures in this field is the most significant mission for us.
List of papers published in 2022
Journal
1. Mizusawa J, Ohba A, Ozaka M, Katayama H, Okusaka T, Kobayashi S, Ikeda M, Terashima T, Sasahira N, Okano N, Miki I, Kaneko T, Mizuno N, Todaka A, Furukawa M, Kajiura S, Kataoka T, Fukuda H, Furuse J, Ueno M, Hepatobiliary and Pancreatic Oncology Group of Japan Clinical Oncology Group. Protocol of a randomized phase II/III study of gemcitabine plus nab-paclitaxel combination therapy versus modified FOLFIRINOX versus S-IROX for metastatic or recurrent pancreatic cancer: JCOG1611 (GENERATE). Japanese Journal of Clinical Oncology, 53:80-84, 2023
2. Ozaka M, Nakachi K, Kobayashi S, Ohba A, Imaoka H, Terashima T, Ishii H, Mizusawa J, Katayama H, Kataoka T, Okusaka T, Ikeda M, Sasahira N, Miwa H, Mizukoshi E, Okano N, Mizuno N, Yamamoto T, Komatsu Y, Todaka A, Kamata K, Furukawa M, Fujimori N, Katanuma A, Takayama Y, Tsumura H, Fukuda H, Ueno M, Furuse J. A randomised phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel for locally advanced pancreatic cancer (JCOG1407). European journal of cancer (Oxford, England : 1990), 181:135-144, 2023
3. Matsuzaki J, Kato K, Oono K, Tsuchiya N, Sudo K, Shimomura A, Tamura K, Shiino S, Kinoshita T, Daiko H, Wada T, Katai H, Ochiai H, Kanemitsu Y, Takamaru H, Abe S, Saito Y, Boku N, Kondo S, Ueno H, Okusaka T, Shimada K, Ohe Y, Asakura K, Yoshida Y, Watanabe SI, Asano N, Kawai A, Ohno M, Narita Y, Ishikawa M, Kato T, Fujimoto H, Niida S, Sakamoto H, Takizawa S, Akiba T, Okanohara D, Shiraishi K, Kohno T, Takeshita F, Nakagama H, Ota N, Ochiya T. Prediction of tissue-of-origin of early stage cancers using serum miRNomes. JNCI cancer spectrum, 7:pkac080, 2023
4. Takeshita K, Hijioka S, Nagashio Y, Maruki Y, Kawasaki Y, Maehara K, Murashima Y, Okada M, Ikeda G, Yamada N, Takasaki T, Agarie D, Hara H, Hagiwara Y, Okamoto K, Yamashige D, Ohba A, Kondo S, Morizane C, Ueno H, Saito Y, Ohe Y, Okusaka T. Diagnostic Ability of Endoscopic Ultrasound-Guided Tissue Acquisition Using 19-Gauge Fine-Needle Biopsy Needle for Abdominal Lesions. Diagnostics (Basel, Switzerland), 13:450, 2023
5. Doi T, Shitara K, Kojima T, Kuboki Y, Matsubara N, Bando H, Yoh K, Naito Y, Hirai H, Kurokawa Y, Kato T, Morizane C. Phase I study of the irreversible fibroblast growth factor receptor 1-4 inhibitor futibatinib in Japanese patients with advanced solid tumors. Cancer science, 114:574-585, 2023
6. Ikeda G, Hijioka S, Nagashio Y, Maruki Y, Ohba A, Hisada Y, Yoshinari M, Harai S, Kitamura H, Koga T, Murashima Y, Maehara K, Okada M, Yamashige D, Okamoto K, Hara H, Hagiwara Y, Agarie D, Takasaki T, Takeshita K, Kawasaki Y, Kondo S, Morizane C, Ueno H, Hiraoka N, Yatabe Y, Saito Y, Iwakiri K, Okusaka T. Fine-needle biopsy with 19G needle is effective in combination with endoscopic ultrasound-guided tissue acquisition for genomic profiling of unresectable pancreatic cancer. Digestive endoscopy, 35:124-133, 2023
7. Hijioka S, Sakamoto Y, Nagashio Y, Maruki Y, Okusaka T, Saito Y. Novel and safe plastic stent exchange method after endoscopic ultrasound-guided hepaticogastrostomy with incomplete fistula (side hole method). Endoscopy, 55:E24-E25, 2023
8. Hijioka S, Sakamoto Y, Nagashio Y, Maruki Y, Okusaka T, Saito Y. Troubleshooting for endoscopic ultrasound-guided hepaticogastrostomy stent migration: Additional stenting by the partial stent-in-stent method. Endoscopy, 55:E122-E124, 2023
9. Maehara K, Hijioka S, Kawasaki Y, Tamada K, Okusaka T, Saito Y. A novel triple stenting in the treatment of post-choledochojejunostomy reflux cholangitis. Endoscopy, 55:E191-E193, 2023
10. Takeshita K, Hijioka S, Maehara K, Maruki Y, Nagashio Y, Okusaka T, Saito Y. Bile duct radiofrequency ablation for a residual adenoma after endoscopic papillectomy. Endoscopy, 55:E185-E188, 2023
11. Kawasaki Y, Hijioka S, Takeshita K, Tamada K, Okusaka T, Saito Y. Endoscopic ultrasound-guided choledochojejunostomy using a forward-viewing echoendoscopic saddle-cross technique. Endoscopy, 55:E233-E235, 2023
12. Furuse J, Ueno M, Ikeda M, Okusaka T, Teng Z, Furuya M, Ioka T. Liposomal irinotecan with fluorouracil and leucovorin after gemcitabine-based therapy in Japanese patients with metastatic pancreatic cancer: additional safety analysis of a randomized phase 2 trial. Japanese journal of clinical oncology, 53:130-137, 2023
13. Tomimaru Y, Eguchi H, Inoue Y, Nagakawa Y, Ohba A, Takami H, Unno M, Yamamoto T, Kawakatsu S, Hayashi T, Higuchi R, Kitagawa H, Hattori S, Fujii T, Hirooka Y, Igarashi H, Kitano M, Kuroki T, Masamune A, Shimizu Y, Tani M, Tanno S, Tsuji Y, Yamaue H, Satoi S, Takeyama Y. Impact of S-1 adjuvant chemotherapy longer than 6 months on survival in patients with resected pancreatic cancer: a nationwide survey by the Japan Pancreas Society based on real-world data. Cancer, 129:728-739, 2023
14. Takeshita K, Hijioka S, Kawasaki Y, Maruki Y, Nagashio Y, Okusaka T, Saito Y. Endoscopic ultrasound-guided hepaticojejunostomy for drainage of the right posterior hepatic duct enabled total liver drainage. Endoscopy, 55:E346-E348, 2023
15. Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, Abrams TA, Furuse J, Kelley RK, Cassier PA, Klümpen HJ, Chang HM, Chen LT, Tabernero J, Oh DY, Mahipal A, Moehler M, Mitchell EP, Komatsu Y, Masuda K, Ahn D, Epstein RS, Halim AB, Fu Y, Salimi T, Wacheck V, He Y, Liu M, Benhadji KA, Bridgewater JA. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. The New England journal of medicine, 388:228-239, 2023
16. Nakachi K, Ikeda M, Konishi M, Nomura S, Katayama H, Kataoka T, Todaka A, Yanagimoto H, Morinaga S, Kobayashi S, Shimada K, Takahashi Y, Nakagohri T, Gotoh K, Kamata K, Shimizu Y, Ueno M, Ishii H, Okusaka T, Furuse J. Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet (London, England), 401:195-203, 2023
17. Takeshita K, Hijioka S, Nagashio Y, Maruki Y, Ohba A, Kawasaki Y, Hisada Y, Yoshinari M, Harai S, Kitamura H, Koga T, Maehara K, Murashima Y, Yamada N, Okada M, Takasaki T, Agarie D, Hara H, Hagiwara Y, Okamoto K, Yamashige D, Kondo S, Morizane C, Ueno H, Saito Y, Okusaka T. Usefulness of a laser-cut covered metal stent with a 7F delivery sheath in endoscopic ultrasound-guided biliary drainage without fistula dilation. Endoscopy international open, 11:E97-E104, 2023
18. Umemoto K, Sunakawa Y, Ueno M, Furukawa M, Mizuno N, Sudo K, Kawamoto Y, Kajiwara T, Ohtsubo K, Okano N, Matsuhashi N, Itoh S, Matsumoto T, Shimizu S, Otsuru T, Hasegawa H, Okuyama H, Ohama H, Moriwaki T, Ohta T, Odegaard JI, Nakamura Y, Bando H, Yoshino T, Ikeda M, Morizane C. Clinical significance of circulating-tumour DNA analysis by metastatic sites in pancreatic cancer. British journal of cancer, 128:1603-1608, 2023
19. Tanaka J, Nakagawa T, Harada K, Morizane C, Tanaka H, Shiba S, Ohba A, Hijioka S, Takai E, Yachida S, Kamura Y, Ishida T, Yokoi T, Uematsu C. Efficient and accurate KRAS genotyping using digital PCR combined with melting curve analysis for ctDNA from pancreatic cancer patients. Scientific reports, 13:3039, 2023
20. Takahara N, Nakai Y, Isayama H, Sasaki T, Morine Y, Watanabe K, Ueno M, Ioka T, Kanai M, Kondo S, Okano N, Koike K. A prospective multicenter phase II study of FOLFIRINOX as a first-line treatment for patients with advanced and recurrent biliary tract cancer. Investigational new drugs, 41:76-85, 2023
21. Ohba A, Morizane C, Ueno M, Kobayashi S, Kawamoto Y, Komatsu Y, Ikeda M, Sasaki M, Okano N, Furuse J, Hiraoka N, Yoshida H, Kuchiba A, Sadachi R, Nakamura K, Matsui N, Nakamura Y, Okamoto W, Yoshino T, Okusaka T. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future oncology (London, England), 18:2351-2360, 2022
22. Kaku S, Horinouchi H, Watanabe H, Yonemori K, Okusaka T, Boku N, Yamazaki N, Kawai A, Ohe Y, Kusumoto M. Incidence and prognostic factors in severe drug-induced interstitial lung disease caused by antineoplastic drug therapy in the real world. Journal of cancer research and clinical oncology, 148:1737-1746, 2022
23. Ida H, Koyama T, Mizuno T, Sunami K, Kubo T, Sudo K, Tao K, Hirata M, Yonemori K, Kato K, Okusaka T, Ohe Y, Matsui Y, Yamazaki N, Ogawa C, Kawai A, Narita Y, Esaki M, Yamamoto N. Clinical utility of comprehensive genomic profiling tests for advanced or metastatic solid tumor in clinical practice. Cancer science, 113:4300-4310, 2022
24. Suzuki K, Yamaguchi T, Kohda M, Tanaka M, Takemura H, Wakita M, Tabe Y, Kato S, Nasu M, Hashimoto T, Mine S, Serizawa N, Tomishima K, Nagahara A, Matsuda T, Yamaji T, Tsugane S, Saito Y, Daiko H, Yoshikawa T, Kato K, Okusaka T, Ochiya T, Yamamoto Y, Yotsui S, Yamamoto T, Yamasaki T, Miyata H, Yasui M, Omori T, Ohkawa K, Ikezawa K, Nakabori T, Sugimoto N, Kudo T, Yoshida K, Ohue M, Nishizawa T. Establishment of preanalytical conditions for microRNA profile analysis of clinical plasma samples. PloS one, 17:e0278927, 2022
25. Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J. Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA oncology, 8:1447-1455, 2022
26. Matsuoka A, Fujimori M, Narikazu B, Takashima A, Okusaka T, Mori K, Akechi T, Shimazu T, Okizaki A, Miyaji T, Majima Y, Nagashima F, Uchitomi Y. Geriatric assessment and management with question prompt list using a web-based application for elderly patients with cancer (MAPLE) to communicate ageing-related concerns: J-SUPPORT 2101 study protocol for a multicentre, parallel group, randomised controlled trial. BMJ open, 12:e063445, 2022
27. Yamamoto S, Sakakibara N, Hirano H, Morizane C, Honma Y, Hijioka S, Okusaka T, Higashi T, Kawai A. The real-world selection of first-line systemic therapy regimen for metastatic gastroenteropancreatic neuroendocrine neoplasm in Japan. Scientific reports, 12:17601, 2022
28. Harai S, Hijioka S, Maruki Y, Ohba A, Nagashio Y, Okusaka T, Saito Y. Endoscopic ultrasound-guided hepaticoduodenostomy with anterograde stenting for recurrent hepatic hilar obstruction. Endoscopy, 54:E398-E400, 2022
29. Kitamura H, Hijioka S, Nagashio Y, Ban D, Esaki M, Okusaka T, Saito Y. A case of high grade pancreatic intraepithelial neoplasia diagnosed by endoscopic ultrasound-guided fine needle aspiration. Endoscopy, 54:E628-E630, 2022
30. Koga T, Hijioka S, Nagashio Y, Ohba A, Maruki Y, Yoshinari M, Hisada Y, Harai S, Kitamura H, Maehara K, Murashima Y, Kawasaki Y, Kawahara S, Takeshita K, Yamada N, Satake T, Kondo S, Morizane C, Ueno H, Okusaka T, Saito Y. Endoscopic ultrasound-guided choledochoduodenostomy without fistula dilation using a stent with a 5.9-Fr delivery system: Comparison to a conventional procedure with fistula dilation. DEN open, 2:e56, 2022
31. Kawasaki Y, Hijioka S, Maehara K, Tamada K, Okusaka T, Saito Y. Endoscopic ultrasound-guided intra-afferent loop entero-enterostomy using a forward-viewing echoendoscope and insertion of a metal stent. Endoscopy, 54:E815-E817, 2022
32. Kawasaki Y, Hijioka S, Nagashio Y, Ohba A, Maruki Y, Maehara K, Yoshinari M, Hisada Y, Harai S, Kitamura H, Murashima Y, Koga T, Kawahara S, Kondo S, Morizane C, Ueno H, Ushio J, Tamada K, Sugawara S, Sone M, Takamoto T, Nara S, Ban D, Esaki M, Arai Y, Shimada K, Saito Y, Okusaka T. A novel endoscopic technique using fully covered self-expandable metallic stents for benign strictures after hepaticojejunostomy: the saddle-cross technique (with video). Surgical endoscopy, 36:9001-9010, 2022
33. Takai E, Nakamura H, Chiku S, Kubo E, Ohmoto A, Totoki Y, Shibata T, Higuchi R, Yamamoto M, Furuse J, Shimizu K, Takahashi H, Morizane C, Furukawa T, Yachida S. Whole-exome Sequencing Reveals New Potential Susceptibility Genes for Japanese Familial Pancreatic Cancer. Annals of surgery, 275:e652-e658, 2022
34. Yanai Y, Makihara RA, Matsunaga N, Shimizu R, Tominaga S, Hoshino S, Nishibuchi Y, Maruki Y, Ohba A, Shimizu K, Okusaka T. A feasibility study of a peer discussion group intervention for patients with pancreatobiliary cancer and their caregivers. Palliative & supportive care, 20:527-534, 2022
35. Sato A, Fujimori M, Shirai Y, Umezawa S, Mori M, Jinno S, Umehashi M, Okamura M, Okusaka T, Majima Y, Miyake S, Uchitomi Y. Assessing the need for a question prompt list that encourages end-of-life discussions between patients with advanced cancer and their physicians: A focus group interview study - ERRATUM. Palliative & supportive care, 20:618-620, 2022
36. Masui T, Ito T, Komoto I, Kojima S, Kasai Y, Tanabe M, Hara K, Hirano S, Okusaka T, Ichikawa Y, Kinugasa Y, Kokudo N, Kudo A, Sakurai A, Sugihara K, Date H, Haruma K, Hijioka S, Hirata K, Yamano H, Sakamine M, Kikuchi T, Fukushima M, Imamura M, Uemoto S. Nationwide registry for patients with neuroendocrine neoplasm of pancreas, gastrointestinal tract, lungs, bronchi, or thymus in Japan. International journal of clinical oncology, 27:840-849, 2022
37. Satake T, Maruki Y, Kubo Y, Takahashi M, Ohba A, Nagashio Y, Kondo S, Hijioka S, Morizane C, Ueno H, Okusaka T. Atezolizumab-induced Encephalitis in a Patient with Hepatocellular Carcinoma: A Case Report and Literature Review. Internal medicine (Tokyo, Japan), 61:2619-2623, 2022
38. Yoshinari M, Hijioka S, Okusaka T, Saito Y. Endoscopic ultrasonography-guided hepaticogastrostomy with parenchymal metal stent placement. Endoscopy, 54:E719-E721, 2022
39. Motzer RJ, Taylor MH, Evans TRJ, Okusaka T, Glen H, Lubiniecki GM, Dutcus C, Smith AD, Okpara CE, Hussein Z, Hayato S, Tamai T, Makker V. Lenvatinib dose, efficacy, and safety in the treatment of multiple malignancies. Expert review of anticancer therapy, 22:383-400, 2022
40. Ohashi Y, Ikeda M, Kunitoh H, Sasako M, Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, Ibusuki K, Takita A, Sakon M. One-year incidence of venous thromboembolism, bleeding, and death in patients with solid tumors newly initiating cancer treatment: Results from the Cancer-VTE Registry. Thrombosis research, 213:203-213, 2022
41. Komoto I, Kokudo N, Aoki T, Morizane C, Ito T, Hashimoto T, Kimura W, Inoue N, Hasegawa K, Kondo S, Ueno H, Igarashi H, Oono T, Makuuchi M, Takamoto T, Hirai I, Takeshita A, Imamura M. Phase I/II study of streptozocin monotherapy in Japanese patients with unresectable or metastatic gastroenteropancreatic neuroendocrine tumors. Japanese journal of clinical oncology, 52:716-724, 2022
42. Hijioka S, Morizane C, Takaori K, Okusaka T. Study protocol for a multi-institutional prospective surveillance study among kindreds with familial pancreatic cancer and individuals with hereditary pancreatic cancer syndrome: The Diamond Study. Pancreatology, 22:534-538, 2022
43. Harai S, Hijioka S, Nagashio Y, Ohba A, Maruki Y, Sone M, Saito Y, Okusaka T, Fukasawa M, Enomoto N. Usefulness of the laser-cut, fully covered, self-expandable metallic stent for endoscopic ultrasound-guided hepaticogastrostomy. Journal of hepato-biliary-pancreatic sciences, 29:1035-1043, 2022
44. Kimura K, Kanto T, Shimoda S, Harada K, Kimura M, Nishikawa K, Imamura J, Ogawa E, Saio M, Ikura Y, Okusaka T, Inoue K, Ishikawa T, Ieiri I, Kishimoto J, Todaka K, Kamisawa T. Safety, tolerability, and anti-fibrotic efficacy of the CBP/β-catenin inhibitor PRI-724 in patients with hepatitis C and B virus-induced liver cirrhosis: An investigator-initiated, open-label, non-randomised, multicentre, phase 1/2a study. EBioMedicine, 80:104069, 2022
45. Satake T, Morizane C, Maruki Y, Ohba A, Nagashio Y, Kondo S, Hijioka S, Ueno H, Okusaka T. The influence of UGT1A1 polymorphisms on modified FOLFIRINOX dose in double-variant-type patients with advanced pancreatic cancer. International journal of clinical oncology, 27:1331-1339, 2022
46. Kaku S, Horinouchi H, Watanabe H, Yonemori K, Okusaka T, Boku N, Yamazaki N, Kawai A, Ohe Y, Kusumoto M. Correction to: Incidence and prognostic factors in severe drug-induced interstitial lung disease caused by antineoplastic drug therapy in the real world. Journal of cancer research and clinical oncology, 148:1747, 2022
47. Chan SL, Schuler M, Kang YK, Yen CJ, Edeline J, Choo SP, Lin CC, Okusaka T, Weiss KH, Macarulla T, Cattan S, Blanc JF, Lee KH, Maur M, Pant S, Kudo M, Assenat E, Zhu AX, Yau T, Lim HY, Bruix J, Geier A, Guillén-Ponce C, Fasolo A, Finn RS, Fan J, Vogel A, Qin S, Riester M, Katsanou V, Chaudhari M, Kakizume T, Gu Y, Porta DG, Myers A, Delord JP. A first-in-human phase 1/2 study of FGF401 and combination of FGF401 with spartalizumab in patients with hepatocellular carcinoma or biomarker-selected solid tumors. Journal of experimental & clinical cancer research, 41:189, 2022
48. Okusaka T, Kudo M, Ikeda K, Ikeda M, Okita K, Sugawara M, Tamai T, Ren M, Saito K, Kumada H. Impact of bodyweight-based starting doses on the safety and efficacy of lenvatinib in primarily Japanese patients with hepatocellular carcinoma. Hepatology research, 52:784-793, 2022
49. Okusaka T. Treatment for postoperative recurrence of pancreatic cancer: a narrative review. Chinese clinical oncology, 11:19, 2022
50. Sato A, Fujimori M, Shirai Y, Umezawa S, Mori M, Jinno S, Umehashi M, Okamura M, Okusaka T, Majima Y, Miyake S, Uchitomi Y. Assessing the need for a question prompt list that encourages end-of-life discussions between patients with advanced cancer and their physicians: A focus group interview study. Palliative & supportive care, 20:564-569, 2022
51. Ohba A, Ueno H, Shiba S, Okano N, Kobayashi T, Nagashima F, Sasahira N, Sasaki M, Imaoka H, Sakamoto Y, Kondo S, Morizane C, Ozaka M, Ikeda M, Furuse J, Okusaka T. Safety and efficacy of S-IROX (S-1, irinotecan and oxaliplatin combination therapy) in patients with advanced pancreatic cancer: A multicenter phase 1b dose-escalation and dose-expansion clinical trial. European journal of cancer (Oxford, England : 1990), 174:40-47, 2022
52. Kubo S, Shinkawa H, Asaoka Y, Ioka T, Igaki H, Izumi N, Itoi T, Unno M, Ohtsuka M, Okusaka T, Kadoya M, Kudo M, Kumada T, Kokudo N, Sakamoto M, Sakamoto Y, Sakurai H, Takayama T, Nakashima O, Nagata Y, Hatano E, Harada K, Murakami T, Yamamoto M. Liver Cancer Study Group of Japan Clinical Practice Guidelines for Intrahepatic Cholangiocarcinoma. Liver cancer, 11:290-314, 2022
53. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Moriguchi M, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Ogasawara S, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver cancer, 11:354-367, 2022
54. Terashima T, Morizane C, Ushiama M, Shiba S, Takahashi H, Ikeda M, Mizuno N, Tsuji K, Yasui K, Azemoto N, Satake H, Nomura S, Yachida S, Sugano K, Furuse J. Germline variants in cancer-predisposing genes in pancreatic cancer patients with a family history of cancer. Japanese journal of clinical oncology, 52:1105-1114, 2022
55. Yoshida Y, Kobayashi S, Ueno M, Morizane C, Tsuji K, Maruki Y, Mori K, Watanabe K, Ohba A, Furuta M, Todaka A, Tsujimoto A, Ozaka M, Okano N, Yane K, Umemoto K, Kawamoto Y, Terashima T, Tsumura H, Doi K, Shioji K, Asagi A, Kojima Y, Suzuki E, Toshiyama R, Furukawa M, Naganuma A, Suzuki R, Miwa H, Ikeda M, Furuse J. Efficacy of chemotherapy for patients with metastatic or recurrent pancreatic adenosquamous carcinoma: A multicenter retrospective analysis. Pancreatology, 22:1159-1166, 2022
56. Satake T, Morizane C, Rikitake R, Higashi T, Okusaka T, Kawai A. The epidemiology of rare types of hepatobiliary and pancreatic cancer from national cancer registry. Journal of gastroenterology, 57:890-901, 2022
57. Hisada Y, Hijioka S, Ikeda G, Maehara K, Hashimoto T, Kitamura H, Harai S, Yoshinari M, Kawasaki Y, Murashima Y, Koga T, Takeshita K, Maruki Y, Ohba A, Nagashio Y, Kondo S, Morizane C, Ueno H, Saito Y, Yatabe Y, Okusaka T. Proportion of unresectable pancreatic cancer specimens obtained by endoscopic ultrasound-guided tissue acquisition meeting the OncoGuide NCC Oncopanel System analysis suitability criteria: a single-arm, phase II clinical trial. Journal of gastroenterology, 57:990-998, 2022
58. McNamara MG, Bridgewater J, Goyal L, Jacobs T, Wagner AD, Goldstein D, Shroff R, Moehler M, Lowery M, Bekaii-Saab T, Kelley RK, Furuse J, Rimassa L, Morizane C, Lamarca A, Hubner R, Knox J, Valle J. What is the gender representation in authorship in later phase systemic clinical trials in biliary tract cancer (BTC)? - a retrospective review of the published literature. BMJ open, 12:e064954, 2022
