Annual Report 2022
Department of Laboratory Medicine
Yasushi Yatabe, Kuniko Sunami, Wataru Takeda, Naoki Maezawa, Chiaki Hayashi, Syunsuke Tezuka, Yoji Hashimoto, Yasuo Shibuki, Hiroki Kakishima, Tomokazu Suzuki, Rie Matsuo, Chiaki Ikeda, Shuji Ota, Arisa Hanai, Satoshi Ito, Yusuke Okui, Sayaka Takeuchi, Kyosuke Tosawa, Mizuho Fujima, Sachiko Kobayashi, Noriko Takahashi, Saori Nakabayashi, Yuki Kase, Shizuka Uyama, Maemi Souma, Tomoe Ito, Kyoko Orihara, Kaori Ueki, Akino Kino, Kana Katsuragi, Ruriko Machida, Haruka Katagiri, Kana Miyajima, Kayo Tei, Nanami Sato, Takako Takada, Kenta Takehara, Kanako Kanaizuka, Hiroshi Chigira, Kaori Yamaguchi, Kyoko Osanai, Moemi Kasane, Hideya Matsubayashi, Yu Aruga, Misato Tsubokura, Yuka Yasuno, Haruki Mitsuya, Kaho Matsui, Nozomi Shishido, Aya Mikami, Saori Kobayashi, Shingo Nakajima, Saeko Shirahama, Mei Fukuhara, Kumi Nakatani, Kazuhiro Yoshida, Hiyori Yatsu, Chika Tokutake, Madoka Kondo, Mayu Takeno, Sakura Ishida, Ayaka Ichikawa, Yusuke Takai, Motoi Miyakoshi, Takashi Kubo, Mayuko Kitami, Satoyo Oda, Shigeru Tamura, Megumi Masuda, Nao Saito, Aya Iwasaki, Hinako Nakamura, Shie Yoda, Mayu Shimono, Nanako Fujita, Moe Kato, Takeshi Ogasawara, Yu Kawajiri, Kazui Taniguchi, Rio Yamauchi, Yuka Hirano, Yoshiko Shibata, Ritsuko Toyama, Chieko Nozawa, Kazue Naoi, Kinue Tsubokawa, Kozaburo Endo, Hiromichi Matsushita
Introduction
The Department of Laboratory Medicine obtained ISO 15189 accreditation, which is an international standard that specifies requirements for quality and competence specific to clinical laboratories, as of September 2012, and has maintained the accreditation ever since.
The number of test requests to the department in FY2022 was 7,283,178, an increase of 113.6% from FY2020, which was significantly affected by the COVID-19 pandemic, and has recovered to the level before the COVID-19 disaster (Table 1).
The Team and What We Do
The Biochemical and General Testing section is actively rotating staff between biochemistry and immunological testing subsections with the aim of improving operational efficiency, and is responding to the increasing number of test requests and clinical trial-related demands.
The Hematology Testing section offers a high-sensitive analysis of minimal/measurable residual diseases in hematopoietic malignancies, and examines many trace-amount specimens such as spinal fluid.
The Microbiology Testing section continuously serves PCR testing for COVID-19 and supports surgical and medical activities in the hospital.
The Genetic Testing section offers cancer genome profiling tests, including liquid biopsy-based tests that are newly covered by insurance in FY2021, and have been conducting more expert panels than ever before (Table 2).
The Blood Collection section is striving to reduce the waiting time for blood collection by setting a goal of "Eighty-five percent of patients wait less than 20 minutes for blood collection", while placing the highest priority on medical safety.
Table 1. Number of clinical tests performed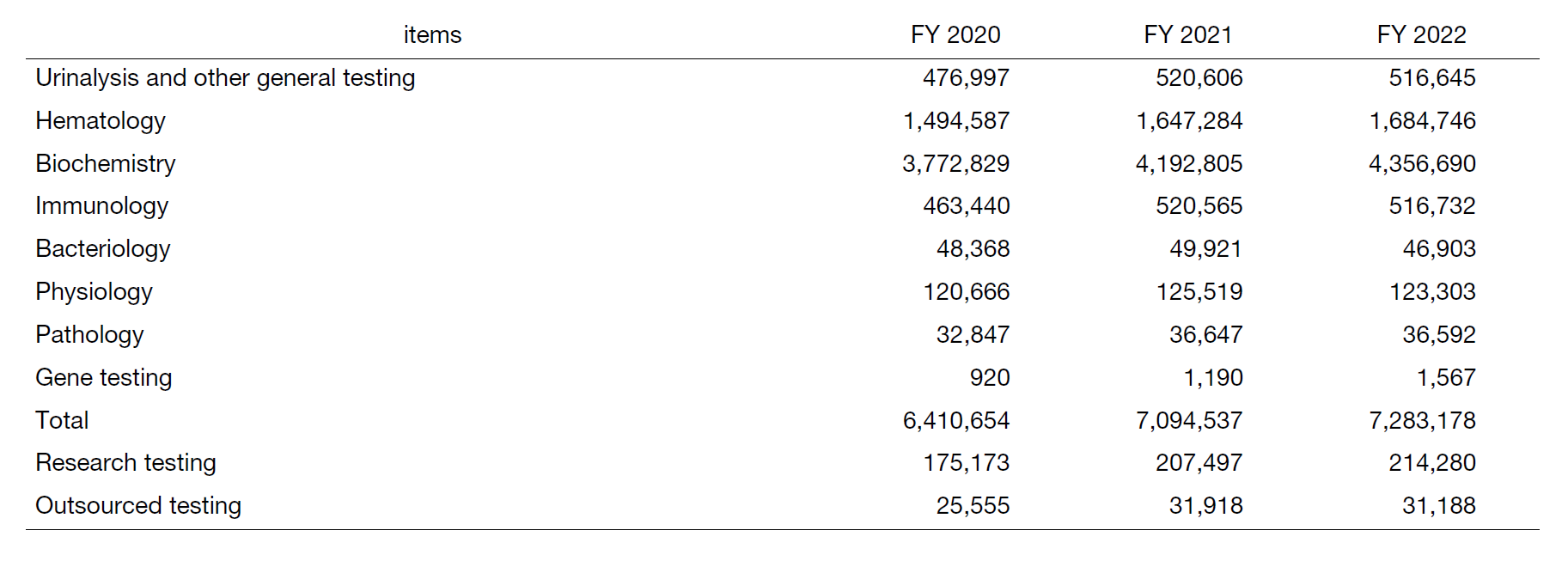

Table 2. Number of sessions and cancer genomic profiling tests in Expert panel meeting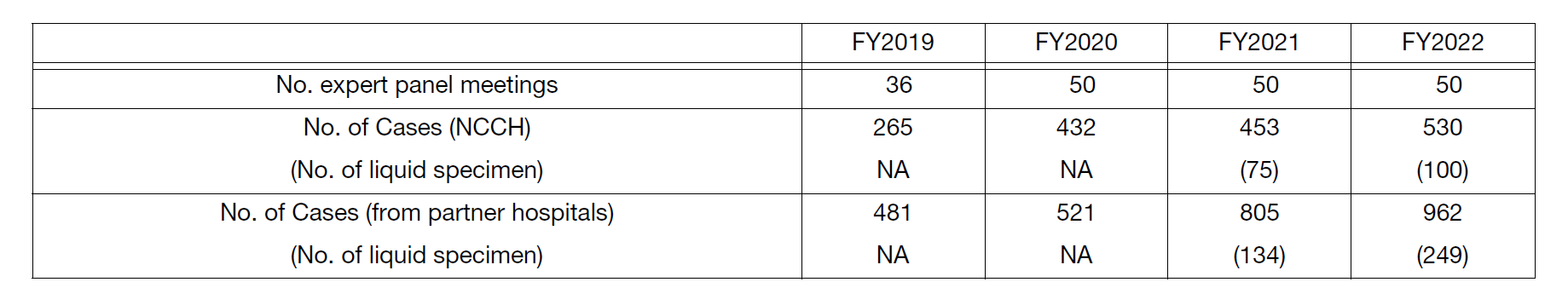

The Blood Transfusion Testing and Cell Processing section is working to improve operational efficiency by introducing automated blood typing and automated ascites fluid processing for Cell-free and Concentrated Ascites Reinfusion Therapy (CART), while also responding to the increasing demands of chimeric antigen receptor-expressing T (CAR-T) cell therapy.
The Physiology Testing section is actively involved in evaluating cardiotoxicity caused by anticancer drugs and diagnosing hepatic sinusoidal obstruction syndrome/vascular occlusion of the central hepatic vein (SOS/VOD) after hematopoietic stem cell transplantation.
The Pathology section continues to process specimens even on weekends and has automated the preparation of liquid-based cytology (LBC) specimens in the gynecology field to further improve operational efficiency.
Research Activities
The Department of Laboratory Medicine has been conducting basic and clinical research in various fields on factors affecting test accuracy, and has reported findings from daily practice and observation. We are studying the stability and clinical usefulness of tumor marker tests and blood coagulation measurement devices in collaboration with clinical departments and manufacturers. Regarding cancer genome profiling tests, the Genetic Testing section is conducting clinical research for the development of new cancer profiling tests and clinical implementation of whole genome analysis as the secretariat of the TOP-GEAR project, a comprehensive genome analysis study in the National Cancer Center. As for blood transfusion and cell therapy, we are cooperating in clinical research conducted by many clinical departments in the hospital, including research on CART.
Education
In accordance with ISO 15189 education, training, and training procedures, the department fosters personnel who can reliably provide quality-assured and accurate test results in each of the section in the laboratory. The department also actively supports academic activities, including taking qualifying examinations, conference presentations, and writing of papers, aiming to train clinical technicians with professional and academic backgrounds.
Our department actively accepts students who wish to become clinical laboratory technician as on-site trainees and appeal to them about clinical laboratory work.
In FY2022, a resident who was aiming to become a certified laboratory doctor completed the required training course and qualified to take the specialist examination.
Future Prospects
As an ISO 15189-accredited testing facility, we guarantee the quality and capabilities meeting international standards and also promote cooperation in clinical trials and clinical studies. In the near future, we will modify the system of the Clinical Laboratory Testing Section for efficient operation of laboratory tests using automated analyzers. Additionally, we will increase the personnel who specialize in cell therapy and flow cytometry analyses through professional education, to expand the capacity of these operations. Regarding cancer genome medicine, we will continue to manage the expert panels, in response to the increased number of cancer genome profiling tests. We will establish a clinical laboratory that can contribute to valuable information from each clinical department as well as provide it ourselves through carrying out these tasks.
List of papers published in 2022
Journal
1. Mizuno S, Ikegami M, Koyama T, Sunami K, Ogata D, Kage H, Yanagaki M, Ikeuchi H, Ueno T, Tanikawa M, Oda K, Osuga Y, Mano H, Kohsaka S. High-Throughput Functional Evaluation of MAP2K1 Variants in Cancer. Molecular cancer therapeutics, 22:227-239, 2023
2. Makise N, Shimoi T, Sunami K, Aoyagi Y, Kobayashi H, Tanaka S, Kawai A, Yonemori K, Ushiku T, Yoshida A. Loss of H3K27 trimethylation in a distinct group of de-differentiated chordoma of the skull base. Histopathology, 82:420-430, 2023
3. Omura T, Takahashi M, Ohno M, Miyakita Y, Yanagisawa S, Tamura Y, Kikuchi M, Kawauchi D, Nakano T, Hosoya T, Igaki H, Satomi K, Yoshida A, Sunami K, Hirata M, Shimoi T, Sudo K, Okuma HS, Yonemori K, Suzuki H, Ichimura K, Narita Y. Clinical Application of Comprehensive Genomic Profiling Tests for Diffuse Gliomas. Cancers, 14:2454, 2022
4. Ida H, Koyama T, Mizuno T, Sunami K, Kubo T, Sudo K, Tao K, Hirata M, Yonemori K, Kato K, Okusaka T, Ohe Y, Matsui Y, Yamazaki N, Ogawa C, Kawai A, Narita Y, Esaki M, Yamamoto N. Clinical utility of comprehensive genomic profiling tests for advanced or metastatic solid tumor in clinical practice. Cancer science, 113:4300-4310, 2022
5. Tsubokura M, Adegawa Y, Kojima M, Tanosaki R, Ohtake R, Kase Y, Iwashita N, Kasane M, Nakabayashi S, Takeuchi S, Kato K, Boku N, Kanemitsu Y, Okusaka T, Fujimoto H, Yonemori K, Ishiki H, Kawamura K, Satomi E, Matsushita H. Adverse effects of cell-free and concentrated ascites reinfusion therapy for malignant ascites: a single-institute experience. BMC cancer, 22:268, 2022
6. Imai M, Nakamura Y, Sunami K, Kage H, Komine K, Koyama T, Amano T, Ennishi D, Kanai M, Kenmotsu H, Maeda T, Morita S, Sakai D, Bando H, Makiyama A, Suzuki T, Hirata M, Kohsaka S, Tsuchihara K, Naito Y, Yoshino T. Expert panel consensus recommendations on the use of circulating tumor DNA assays for patients with advanced solid tumors. Cancer science, 113:3646-3656, 2022
7. Sunami K, Naito Y, Komine K, Amano T, Ennishi D, Imai M, Kage H, Kanai M, Kenmotsu H, Koyama T, Maeda T, Morita S, Sakai D, Kohsaka S, Tsuchihara K, Saigusa Y, Yoshino T. Chronological improvement in precision oncology implementation in Japan. Cancer science, 113:3995-4000, 2022
8. Hamamoto R, Koyama T, Kouno N, Yasuda T, Yui S, Sudo K, Hirata M, Sunami K, Kubo T, Takasawa K, Takahashi S, Machino H, Kobayashi K, Asada K, Komatsu M, Kaneko S, Yatabe Y, Yamamoto N. Introducing AI to the molecular tumor board: one direction toward the establishment of precision medicine using large-scale cancer clinical and biological information. Experimental hematology & oncology, 11:82, 2022
9. Naito Y, Sunami K, Kage H, Komine K, Amano T, Imai M, Koyama T, Ennishi D, Kanai M, Kenmotsu H, Maeda T, Morita S, Sakai D, Watanabe K, Shirota H, Kinoshita I, Yoshioka M, Mamesaya N, Ito M, Kohsaka S, Saigusa Y, Yamamoto K, Hirata M, Tsuchihara K, Yoshino T. Concordance Between Recommendations From Multidisciplinary Molecular Tumor Boards and Central Consensus for Cancer Treatment in Japan. JAMA network open, 5:e2245081, 2022
10. Kojima N, Arai Y, Satomi K, Kubo T, Matsushita Y, Mori T, Matsushita H, Ushijima T, Yatabe Y, Shibata T, Yonemori K, Ichimura K, Ichikawa H, Kawai A, Yoshida A. Co-expression of ERG and CD31 in a subset of CIC-rearranged sarcoma: a potential diagnostic pitfall. Modern pathology, 35:1439-1448, 2022
11. Yoshizawa A, Hiroshima K, Takenaka A, Haba R, Kawahara K, Minami Y, Kakinuma H, Shibuki Y, Miyake S, Kajio K, Kiyonaga K, Nagatomo M, Nishimura S, Mano M, Matsubayashi J, Motoi N, Nagao T, Nakatsuka SI, Yoshida T, Satoh Y. Cytology Reporting System for Lung Cancer from the Japan Lung Cancer Society and the Japanese Society of Clinical Cytology: An Extensive Study Containing More Benign Lesions. Acta cytologica, 66:124-133, 2022
