Annual Report 2022
Department of International Clinical Development
Kenichi Nakamura, Kan Yonemori, Kayo Motono, Yuuka Okamoto, Tatsunori Shimoi, Seiichiro Abe, Hiroshi Igaki, Miyuki Sone, Shunsuke Tsukamoto, Hiroko Nakahama, Tomomi Hata, Yoichi Osato, Miho Nakajima, Kazuko Ohara, Hitomi Okuma, Yuta Maruki, Yusuke Okuma, Masamichi Takahashi, Koichi Ogura, Chiharu Mizoguchi, Rina Inagaki, Kazuki Sudo, Shinji Kohsaka, Yuki Kojima, Sho Shiino, Hiroshi Yoshida, Keisuke Watanabe, Tomohiro Matsuda, Mitsumi Terada, Nobuko Ushirozawa, Hisahiro Itoh, Kumiko Yanagihara, Hitomi Otsuka, Jalutangsee Sukanya, Patchara Promsurin
Introduction
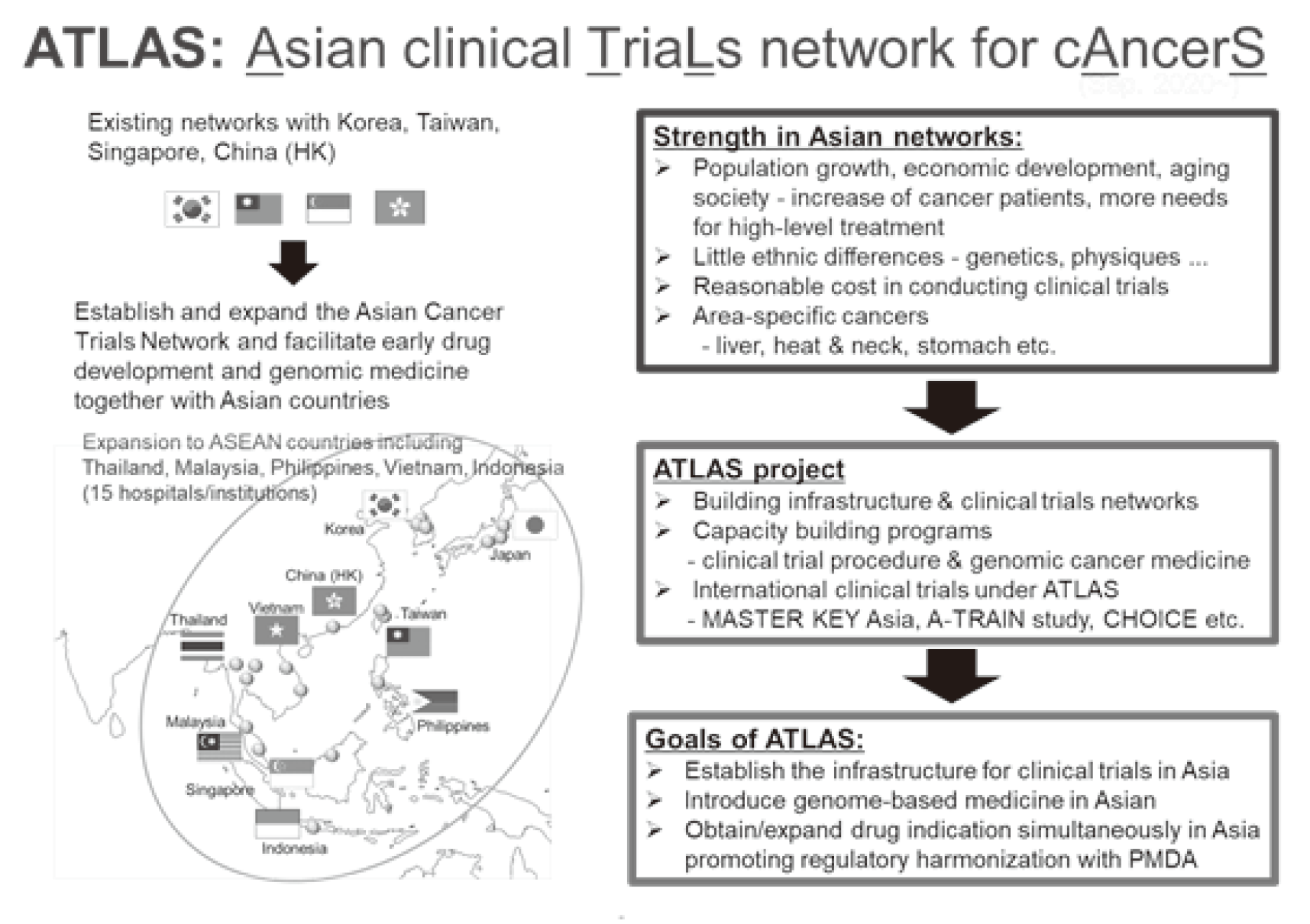
The Department of International Clinical Development (DICD) was established at the National Cancer Center Hospital in 2020 to accelerate international research, education, and treatment under an integrated vision, and to promote the Asian Clinical Trials Network project (ATLAS project).
The Team and What We Do
(International Medical Care Section)
This section focuses on improving the quality of care and increasing the number of overseas patients at the NCCH. In FY2022, this section developed an institutional vision of international medical care, introduced the NCCH’s international medical care activities to overseas hospitals, established online second opinion system, and organized the flow of accepting overseas patients.
(International Professional Education Section)
In FY2022, as part of the ATLAS project, more than 50 English contents were published on the ICR website, and the section solicited more overseas users for the educational contents. In collaboration with PMDA, a Multi-regional Clinical Trials Seminar in January 2023 and a GCP Inspection Seminar in February 2023 were held for participants from overseas regulatory authorities and overseas medical institutions.
(International Research and Development Section)
In FY2022, the MASTER KEY project, a platform trial for rare cancers, was further expanded to Asian countries. Two new international collaborative studies, HARMONY and CHOCE, were launched. The section also continued internal/external call for international clinical trials in Asia, and the section started preparation of a new Asian clinical trial of Software as a Medical Device for colorectal cancer.
(International Translational Research Section)
In FY2022, patient accrual of the A-TRAIN study was continued. This is a TR study of liquid biopsy in which 10 Asian countries will participate. In addition, the section supported HARMONY study, an observational study using MRD technique in the field of breast cancer.
(Asian Partnerships Section)
As part of the ATLAS project, the NCC Asian Partnership Office has been established in Bangkok, Thailand, which will have a local coordination function for international clinical studies and strengthen the network with local investigators, governments and regulatory authorities.
Research Activities
The ATLAS Symposium was held in April 2022 to disseminate the research activities of the ATLAS project to domestic and international participants, ongoing international collaborative trials, and regulatory harmonization with Asian countries. In addition, several presentations on the ATLAS project were made at conferences such as ARO Council, Japan Cancer Association, DIA Japan, ASCOMOS Malaysia, CRM Connect Malaysia, and DIA China.
Clinical Trials
Multiple international clinical studies are ongoing such as: PATHWAY, an investigator-initiated registration-directed trial for breast cancer; MASTER KEY ASIA, a platform study for rare cancer development; and the A-TRAIN study, a TR study of liquid biopsy with 10 Asian countries; CHOICE, translational research of genomic medicine for cholangiocarcinoma; HARMONY, a translational research with MRD technique for breast cancer. Another new Asian clinical trial is under preparation, which aims at establishing standard colorectal cancer screening procedures using a newly developed Software as a Medical Device.
Education
On-the-job training of international research support staff was made through coordination of international clinical studies. In addition, MRCT seminars and GCP inspection seminars were held jointly with PMDA for overseas regulators and researchers.
Future Prospects
DICD continues to promote the ATLAS Project and increase the number of international collaborative research. To this end, we will continue to call for new international research once every six months both within and outside the hospital. In addition, the contents of the ICR website will be enhanced, and various educational seminars will be held jointly with other Asian countries. As for international medical care, DICD will promote the development of an online second opinion system and build an organization that actively promotes the acceptance of foreign patients.
List of papers published in 2022
Journal
1. Ishiki H, Kikawa Y, Terada M, Mizusawa J, Honda M, Iwatani T, Mizutani T, Mori K, Nakamura N, Miyaji T, Yamaguchi T, Ando M, Nakamura K, Fukuda H, Kiyota N. Patient-reported outcome and quality of life research policy: Japan Clinical Oncology Group (JCOG) policy. Japanese journal of clinical oncology, 53:195-202, 2023
2. Yazaki S, Shimoi T, Yoshida M, Sumiyoshi-Okuma H, Arakaki M, Saito A, Kita S, Yamamoto K, Kojima Y, Nishikawa T, Tanioka M, Sudo K, Noguchi E, Murata T, Shiino S, Takayama S, Suto A, Ohe Y, Fujiwara Y, Yonemori K. Integrative prognostic analysis of tumor-infiltrating lymphocytes, CD8, CD20, programmed cell death-ligand 1, and tertiary lymphoid structures in patients with early-stage triple-negative breast cancer who did not receive adjuvant chemotherapy. Breast cancer research and treatment, 197:287-297, 2023
3. Yazaki S, Salgado R, Shimoi T, Yoshida M, Shiino S, Kaneda T, Kojima Y, Sumiyoshi-Okuma H, Nishikawa T, Sudo K, Noguchi E, Murata T, Takayama S, Suto A, Ohe Y, Yonemori K. Impact of adjuvant chemotherapy and radiotherapy on tumour-infiltrating lymphocytes and PD-L1 expression in metastatic breast cancer. British journal of cancer, 128:568-575, 2023
4. Kojima Y, Sudo K, Yoshida H, Yazaki S, Tokura M, Mizoguchi C, Okuma HS, Kita S, Yamamoto K, Nishikawa T, Noguchi E, Shimoi T, Tanase Y, Uno M, Ishikawa M, Kato T, Koyama K, Kobayashi M, Kakegawa T, Fujiwara Y, Yonemori K. Changes in HER3 expression profiles between primary and recurrent gynecological cancers. Cancer cell international, 23:18, 2023
5. Okuma HS, Yoshida H, Kobayashi Y, Arakaki M, Mizoguchi C, Inagaki L, Voon PJ, Malik Bin Ismail A, Fen Soo Hoo H, Yusak S, Severino B Imasa M, Nguyen Huy T, Thai Anh T, Kohsaka S, Mano H, Yonemori K, Nakamura K, Yatabe Y. Molecular pathology quality control in Southeast Asia: Results of a multiregional quality assurance study from MASTER KEY Asia. Cancer science, 114:2664-2673, 2023
6. Nishikawa T, Hasegawa K, Matsumoto K, Mori M, Hirashima Y, Takehara K, Ariyoshi K, Kato T, Yagishita S, Hamada A, Kawasaki M, Kawashima S, Tomatsuri S, Nagasaka Y, Yoshida H, Machida R, Hirakawa A, Nakamura K, Yonemori K. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. Journal of clinical oncology, 41:2789-2799, 2023
7. Sanomachi T, Okuma HS, Kitadai R, Kawachi A, Yazaki S, Tokura M, Arakaki M, Saito A, Kita S, Yamamoto K, Maejima A, Kojima Y, Nishikawa T, Sudo K, Shimoi T, Noguchi E, Fujiwara Y, Sugino H, Shiino S, Suto A, Yoshida M, Yonemori K. Low HER2 expression is a predictor of poor prognosis in stage I triple-negative breast cancer. Frontiers in oncology, 13:1157789, 2023
8. Sanomachi T, Sumiyoshi Okuma H, Yonemori K. COVID arm that appeared in the contralateral upper extremity after mRNA-1273 booster inoculation. International cancer conference journal, 12:216-219, 2023
9. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe SI, Asamura H. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet (London, England), 399:1607-1617, 2022
10. Takeuchi H, Ito Y, Machida R, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Okuno T, Hironaka S, Nozaki I, Ura T, Chin K, Kojima T, Seki S, Sakanaka K, Fukuda H, Kitagawa Y. A Single-Arm Confirmatory Study of Definitive Chemoradiation Therapy Including Salvage Treatment for Clinical Stage II/III Esophageal Squamous Cell Carcinoma (JCOG0909 Study). International journal of radiation oncology, biology, physics, 114:454-462, 2022
11. Chiba Y, Sudo K, Kojima Y, Okuma H, Kohsaka S, Machida R, Ichimura M, Anjo K, Kurishita K, Okita N, Nakamura K, Kinoshita I, Takahashi M, Matsubara J, Kusaba H, Yonemori K, Takahashi M. A multicenter investigator-initiated Phase 2 trial of E7090 in patients with advanced or recurrent solid tumor with fibroblast growth factor receptor (FGFR) gene alteration: FORTUNE trial. BMC cancer, 22:869, 2022
12. Tomotaka S, Mitsumi T, Ryunosuke M, Tomoko K, Yuta I, Keisuke K, Dai M, Hirokazu N, Lymphoma Study Group of the Japan Clinical Oncology Group. Randomized phase III study of daratumumab versus bortezomib plus daratumumab as maintenance therapy after D-MPB for transplant-ineligible patients with untreated multiple myeloma (JCOG1911, B-DASH study). Japanese Journal of Clinical Oncology, 53:349-354, 2022
13. Omura T, Takahashi M, Ohno M, Miyakita Y, Yanagisawa S, Tamura Y, Kikuchi M, Kawauchi D, Nakano T, Hosoya T, Igaki H, Satomi K, Yoshida A, Sunami K, Hirata M, Shimoi T, Sudo K, Okuma HS, Yonemori K, Suzuki H, Ichimura K, Narita Y. Clinical Application of Comprehensive Genomic Profiling Tests for Diffuse Gliomas. Cancers, 14:2454, 2022
14. Yazaki S, Kojima Y, Yoshida H, Takamizawa S, Kitadai R, Nishikawa T, Shimoi T, Sudo K, Saito A, Okuma HS, Tanioka M, Noguchi E, Uno M, Ishikawa M, Kato T, Fujiwara Y, Ohe Y, Yonemori K. High expression of folate receptor alpha is associated with poor prognosis in patients with cervical cancer. Journal of gynecologic oncology, 33:e82, 2022
15. Takamizawa S, Yazaki S, Kojima Y, Yoshida H, Kitadai R, Nishikawa T, Shimoi T, Sudo K, Okuma HS, Tanioka M, Noguchi E, Uno M, Ishikawa M, Kato T, Fujiwara Y, Yonemori K. High mesothelin expression is correlated with non-squamous cell histology and poor survival in cervical cancer: a retrospective study. BMC cancer, 22:1215, 2022
16. Kojima Y, Shimoi T, Seo T, Yazaki S, Okuya T, Ohtake Y, Okuma HS, Shimomura A, Nishikawa T, Tanioka M, Sudo K, Noguchi E, Tamura K, Yoshida A, Iwata S, Kobayashi E, Kawai A, Fujiwara Y, Yonemori K. Poor Treatment Outcomes with Second-Line Chemotherapy in Advanced Synovial Sarcoma. Oncology, 100:370-375, 2022
17. Takamizawa S, Shimoi T, Yoshida M, Tokura M, Yazaki S, Mizoguchi C, Saito A, Kita S, Yamamoto K, Kojima Y, Sumiyoshi-Okuma H, Nishikawa T, Noguchi E, Sudo K, Yonemori K. Diagnostic value of tumor markers in identifying favorable or unfavorable subsets in patients with cancer of unknown primary: a retrospective study. BMC cancer, 22:412, 2022
18. Rowsell A, Sodergren SC, Vassiliou V, Darlington AS, Guren MG, Alkhaffaf B, Moorbey C, Dennis K, Terada M. Systematic review of health-related quality of life (HRQoL) issues associated with gastric cancer: capturing cross-cultural differences. Gastric cancer, 25:665-677, 2022
