Home > Divisions & Departments > Division of Epidemiology > project > Management of Biological Specimens
Management of Biological Specimens
Management of Biological Specimens
▼ Japan Public Health Center-based Prospective Study (JPHC Study)
The JPHC Study (linked to an external website for NCC) is a large-scale cohort study targeting approximately 140,000 residents aged 40-69 living in the jurisdiction of 11 public health centers in Japan. A baseline survey was conducted in each region from 1990 to 1994, followed by three large-scale surveys conducted at five-year intervals. Further, information on outcomes such as mortality, cancer, and cardiovascular diseases has been collected since the initiation of the study. In our division, we manage and actively utilize biological specimens (blood samples) collected during the baseline survey and the 5-year follow-up survey.
Regarding blood samples, we requested residents to provide their blood samples during health checkups. At the time of blood collection during the checkup, we collected 10 mL of their blood in each blood collection tube including heparin for research purposes and centrifuged them within 12 hours. Subsequently, we aliquoted 1 mL of plasma into each of three freezing preservation tubes and buffy coat (i.e., white blood cell layer) into one freezing preservation tube. Each of these aliquots was stored in a -80℃ ultra-low-temperature freezer.
For further information by region, please click here (linked to an external Japanese website for NCC).
▼ Japan Public Health Center-based Prospective Study for the Next Generation (JPHC-NEXT Study)
The JPHC-NEXT Study (linked to an external website for NCC) is a large-scale cohort study targeting approximately 110,000 residents aged 40-74 living in 8 regions across 7 prefectures in Japan. A baseline survey was conducted in each region from 2011 to 2016, followed by a planned total of three large-scale surveys at five-year intervals. Currently, the study is conducting the third round of the investigation, namely the 10-year follow-up survey. Information on outcomes such as mortality, cancer, and cardiovascular diseases has been collected since the initiation of the study. In our division, we primarily manage and utilize biological specimens (blood and urine samples) collected during each large-scale survey.
The collection of the biological specimens in the baseline survey was carried out during specific health checkups for persons aged 40–74 under the national health insurance system, comprehensive health checkup system (Ningen Dock) at collaborative research institutions, and occasions specifically organized for blood and urine collection of this study. In blood collection, 7 mL of blood was collected in each EDTA-2Na-added vacuum blood collection tube. Following centrifugation, 1 mL of plasma was aliquoted into each of three freezing preservation tubes while buffy coat (i.e., white blood cell layer) and red blood cell layer were each aliquoted into one freezing preservation tube. On the other hand, 4 mL of urine samples was aliquoted into one freezing preservation tube. The aliquoted specimens are stored in a -80℃ ultra-low-temperature freezer promptly on the day of collection.
For detailed procedures and collection status regarding biological specimen collection in the baseline survey, please refer to this document (linked to an external Japanese website for NCC).
▼ National Cancer Center Japan-Screening Cohort Study (JaSCo Study)
Since the establishment of the "Research Center for Cancer Prevention and Screening" in February 2004 at National Cancer Center (now located in the Cancer Screening Center at National Cancer Center Hospital), the institution has been providing several cancer screenings. Additionally, a prospective study aimed at elucidating causes of cancer and developing new screening methods, namely the JaSCo study, (linked to an external website for NCC; https://epi.ncc.go.jp/mstudy/index.html) has also been conducted for individuals aged 40 and above who attend screenings. In our division, we manage and vigorously utilize biological specimens (blood and urine samples) collected during these screenings.
1) First Phase Study
The period from February 2004, when cancer screenings were conducted at the building of the Research Center for Cancer Prevention and Screening, until December 2013 is referred to as the First Phase Study. During this time, nearly 15,000 initial attendees participated in this study, with approximately 6,500 individuals also cooperating in the 5-year follow-up survey at their subsequent screenings. Until January 2009, two EDTA-2Na-added vacuum blood collection tubes were used for each 7 mL blood collection, followed by centrifugation. 1 mL of plasma was aliquoted into each of four freezing preservation tubes and buffy coat (i.e., white blood cell layer) into two freezing preservation tubes. All the aliquoted specimens were promptly stored at -80℃ ultra-low-temperature freezers on the day of collection. From February 2009 onwards, 7 mL of blood was collected in one EDTA-2Na-added vacuum blood collection tube and 9 mL in one vacuum blood collection tube without anticoagulant. Residual urine during screenings was also collected. 1 mL of plasma was aliquoted into each of three freezing preservation tubes, 1mL of serum into each of four tubes, buffy coat into each of two tubes, and urine into each of three tubes. All the tubes were stored at -80℃ ultra-low-temperature freezers on the same day.
2) Second Phase Study
The period from April 2014, when cancer screening started at National Cancer Center Hospital, until the present is referred to as the Second Phase Study. As of the end of March 2023, over 8,000 initial attendees have participated in this study. 7 mL of blood was collected, using each of two vacuum blood collection tubes with EDTA-2Na. Following centrifugation, 1 mL of plasma was aliquoted into each of six freezing preservation tubes while buffy coat was aliquoted into each of two freezing preservation tubes. Just as the First Phase Study, all the aliquoted specimens were promptly stored at -80℃ in an ultra-low-temperature freezer on the collection day.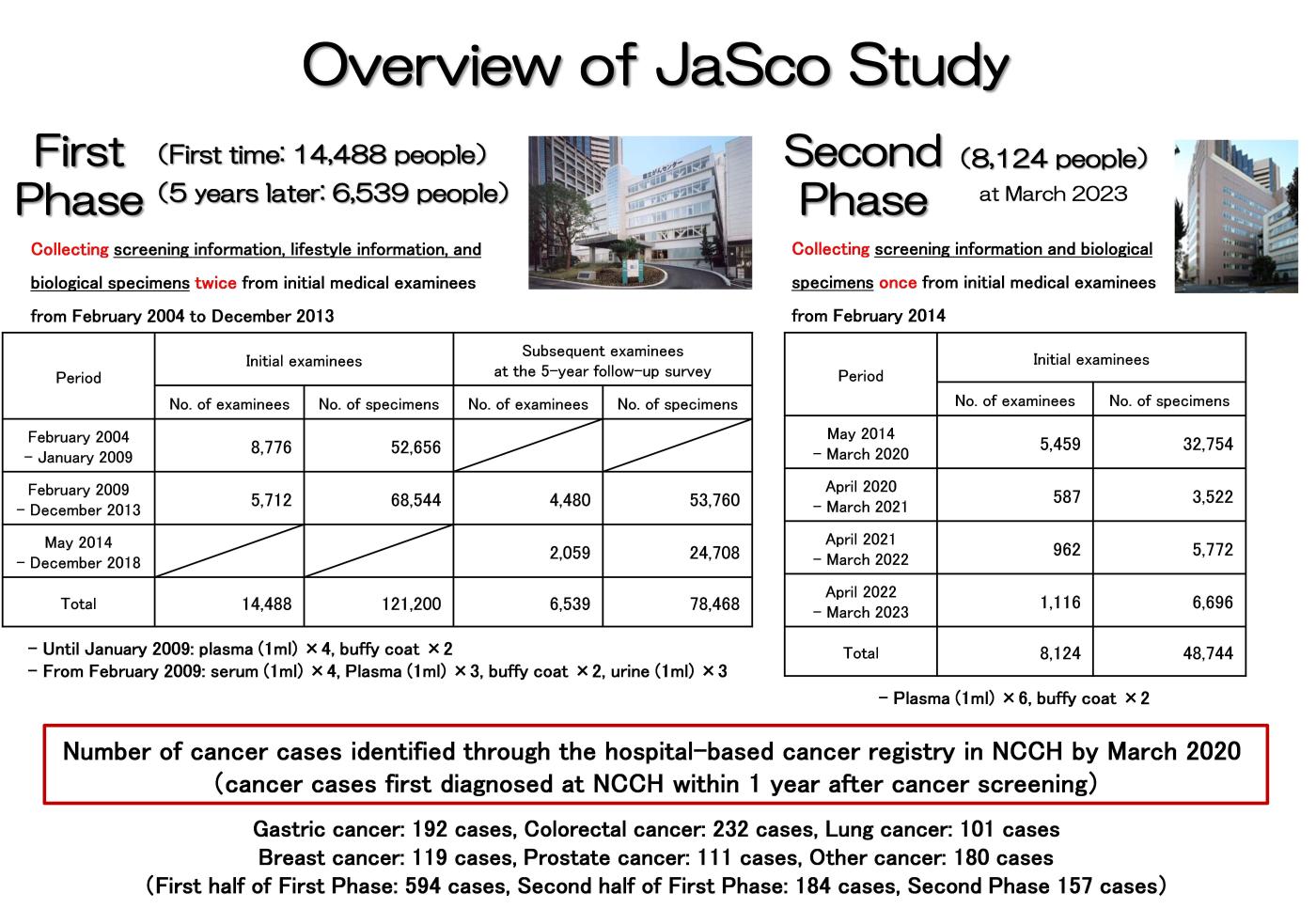
Establishment of Research Infrastructure for Utilization of Biological Specimens
▼ Japan Public Health Center-based Prospective Study (JPHC Study)
In a large-scale cohort study analyzing biological specimens, the exhaustive measurement of all samples under consideration poses substantial challenges in terms of both cost and effort. To address this, research designs that have been proposed focus on a subset of the target population while prioritizing statistical accuracy. Typical examples include nested case-control studies and case-cohort studies. Previously, nested case-control study designs were applied to the analysis of specific cancer sites as individual studies. In recent times, an analytical framework applying a case-cohort study design has been established, considering diverse analytical needs and efficiently obtaining information related to biological specimen measurements. One such example is multiple utilization of omics information such as genomic data.
1) Case-Cohort Study on Overall Cancer Based on Baseline Survey (Study Utilizing Genomic Information)
A case-cohort study on overall cancer was undertaken, utilizing data collected from a baseline survey conducted between 1990 and 1994, involving questionnaires and blood samples from 33,736 participants followed up until the end of 2009. Among 33,736 participants, 3,750 cancer cases were identified through population-based cancer registry, and a subcohort of 13,024 individuals was randomly sampled. Comprehensive genotyping using SNP arrays was performed on DNA samples from both cancer cases and the subcohort. These data have been employed in various ways: 1) genome-wide association study setting cancer and other traits as outcomes, 2) investigation into gene-environment interactions, 3) Mendelian randomization analysis with genomic information, exemplified by genetic polymorphisms, as instrumental variables, and 4) application to predictive models for estimating absolute risk.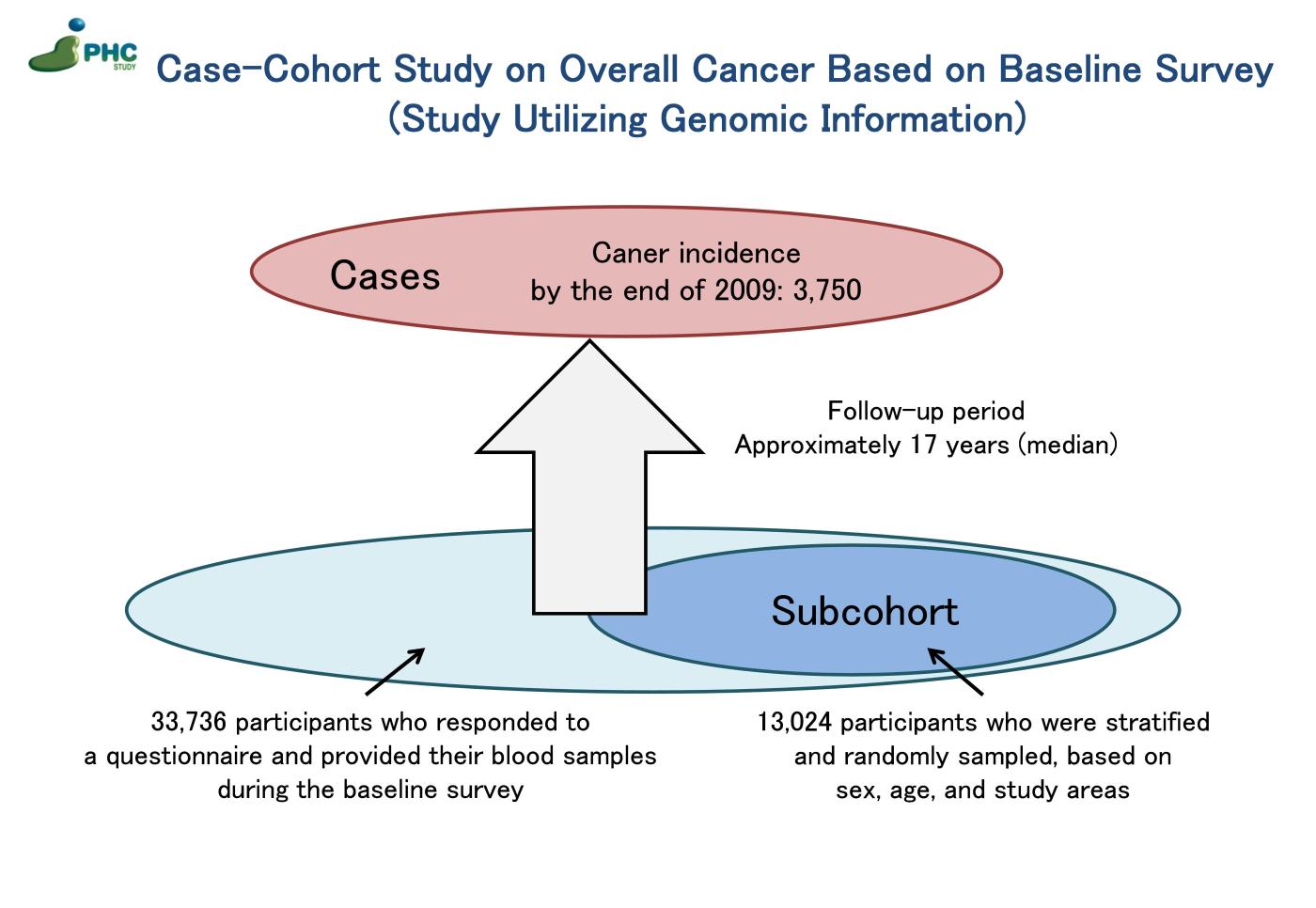 2) Case-Cohort Study on Overall Cancer Based on Baseline Survey (Study Utilizing Plasma)
2) Case-Cohort Study on Overall Cancer Based on Baseline Survey (Study Utilizing Plasma)
Utilizing data collected from a baseline survey conducted between 1990 and 1994, involving questionnaires and plasma samples from 33,736 participants followed up until the end of 2009. Among 33,736 participants, 3,750 cancer cases were identified through population-based cancer registry, and a subcohort of 4,456 individuals was randomly sampled (note: the participants in this subcohort overlap with those selected for the Study Utilizing Genomic Information described above). Plasma samples from both cancer cases and the subcohort were utilized to measure concentrations of blood biomarkers, including 25(OH) vitamin D, C-peptide, glycated albumin, C-reactive protein, and iron metabolism markers.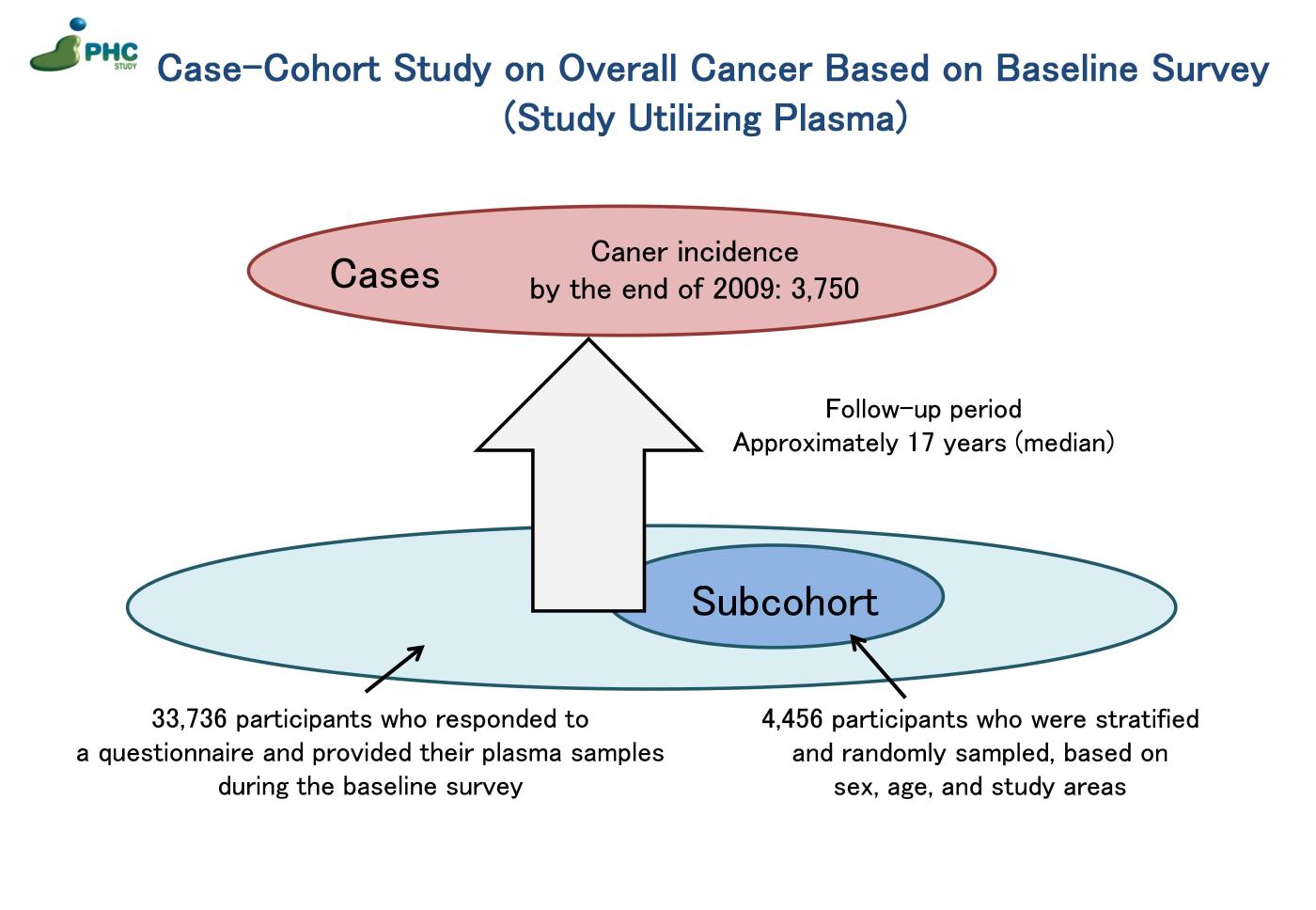
3) Case-Cohort Study on Overall Cancer Based on 5-Year Follow-up Survey (Study Utilizing Genomic Information)
A case-cohort study on overall cancer was conducted, utilizing a 5-year follow-up survey between 1995 and 1998. Among individuals who did not participate in the baseline survey, 10,950 participants responded to a questionnaire and provided their blood samples during the 5-year follow-up survey and were then followed up until the end of 2009. From this population, 1,081 cancer cases were identified through cancer registration, and a subcohort of 4,000 individuals was randomly selected. Comprehensive genotyping using SNP arrays was performed on DNA samples from both cancer cases and the subcohort. This information is utilized in the following ways: conducting analysis in conjunction with the aforementioned “Case-Cohort Study on Overall Cancer Based on the Baseline Survey” and validating results obtained from the same study.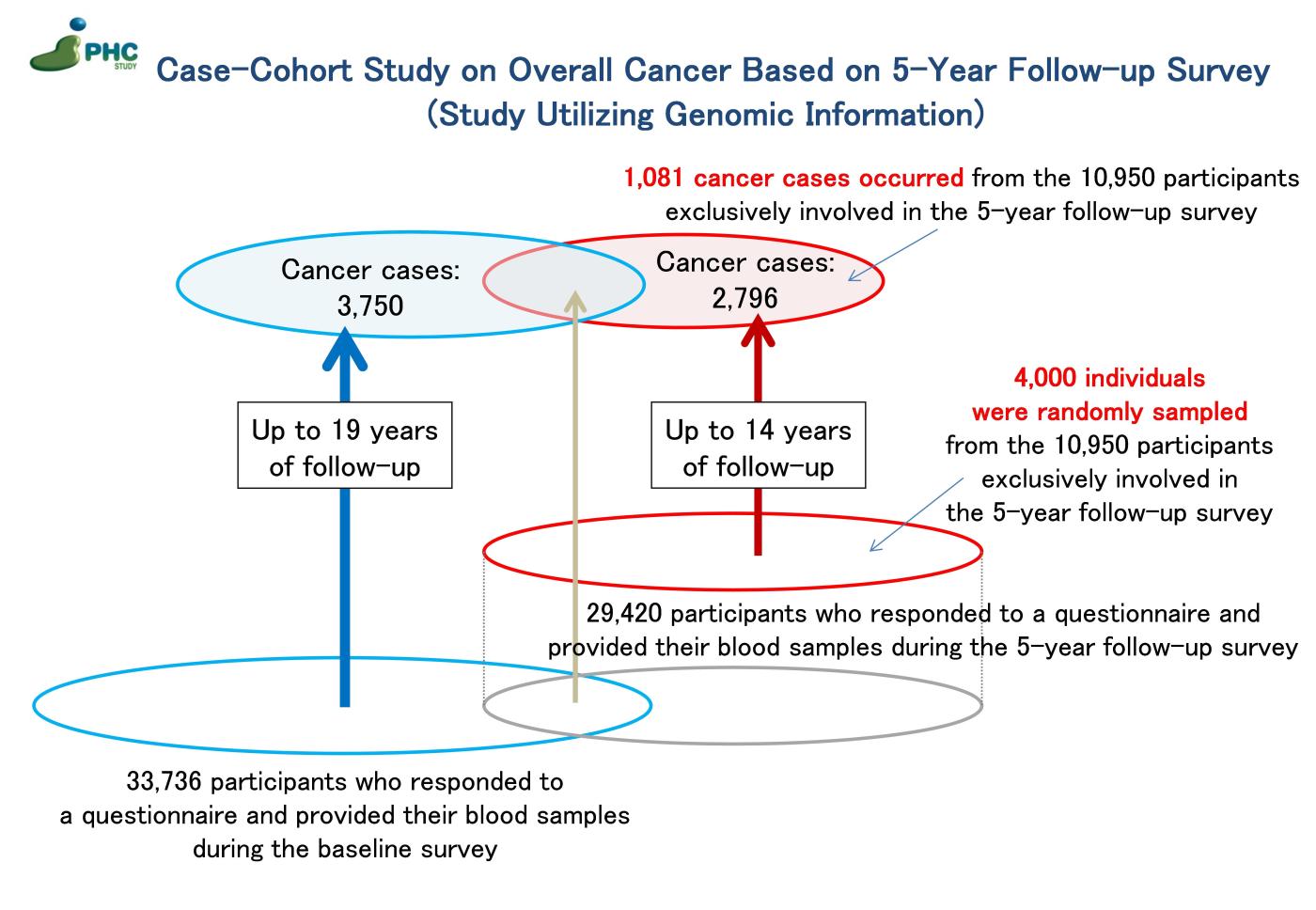
▼ Japan Public Health Center-based Prospective Study for the Next Generation (JPHC-NEXT Study)
1) Whole Genome Sequencing for Cancer Cases
We have initiated whole genome sequencing to explore germline variants in sporadic cancer cases with a scarcity of familial accumulation, which is frequently observed in cohort studies targeting the general population. The analysis focuses on individuals diagnosed with cancer by the end of 2016 among 60,928 participants (26,364 males and 34,564 females) who provided their blood samples during the baseline or 5-year follow-up surveys of the JPHC-NEXT Study.
