Home > Information > press release > Collaborative Research for Advanced Data Linkage between Electronic Medical Records and Clinical Trial Data Collection System
Collaborative Research for Advanced Data Linkage between Electronic Medical Records and Clinical Trial Data Collection System- Expedited Transcription at the Medical Institution and for SDV in the Pharmaceutical Company -
Highlights
- PhambieLINQ® has been confirmed to electronically link adverse events and concomitant medications from the electronic medical record to the EDC, in addition to the existing patient demographics, vital signs, and clinical laboratory tests.
- Data linkage has been shown to reduce the time required for transcription in medical institutions from electronic medical records to EDC and to reduce the number of queries, while pharmaceutical companies have seen a reduction in SDV work time.
- The ability to electronically link electronic medical records to EDC is expected to lead to the enhancement of efficiency and quality of clinical trial operations throughout the entire industry including other medical institutions and pharmaceutical companies.
Summary
TOKYO, June 11, 2024 -- National Cancer Center Hospital East (hereafter, NCCHE), Chugai Pharmaceutical Co., Ltd.(linked at external site) (TOKYO: 4519), and NTT DATA Japan conducted collaborative research on the utilization of clinical data including adverse events and concomitant medications in clinical trials.
Linking clinical data recorded on electronic medical record (EMR) to the electronic data capture system (EDCNote1) of pharmaceutical companies have been widely pursued, but were limited to certain clinical data. In this collaborative research, we utilized PhambieLINQ®Note2 to link expanded variants from clinical data, including adverse events and concomitant medications, required for any clinical trial regardless of the disease. Even on a global level, this is a forward-thinking collaborative research, which will propel the digitalization of clinical trial operations.
In the future, the three parties will continue to work together, so that new drugs can reach patients as quickly as possible by utilizing the results of this collaborative research whilst enhancing the efficiency and quality of clinical trial operations of medical institutions and pharmaceutical companies.
Background
It takes an average of 9 to 17 years to develop a new drug which includes the processes of collection/analysis of clinical data and assessment of efficacy and safety in clinical trialsNote3. Enhancing efficiency while ensuring high quality in collection of clinical data, which is labor-intensive and time-consuming, is challenging for both pharmaceutical companies and medical institutions.
Recently, attempts at linking clinical data electronically from EMR in the medical institutions to EDC in pharmaceutical companies has been drawing attention. However, the practice is not yet common in the industry due to incompatibility between EMR and EDC, as well as variability among products. Especially, data linkage of information regarding adverse events and concomitant medications, which is critical for the safety assessment, is rarely practiced.
NTT DATA is committed to the continuous advancement and provision of the clinical trial DX service PhambieLINQ®, which enables the data linkage between EMR and EDC for any medical institutions and pharmaceutical companies. Currently, three types of clinical data such as patient demographics, clinical laboratory tests, and vital signs, can be linked using PhambieLINQ®; however, other clinical data including adverse events and concomitant medications are also recorded in medical charts. If these clinical data can also be electronically linked to EDC, further enhancement of efficiency and quality can be expected.
NCCHE accelerates medical digitalization by working on structured data management including data collection and use while utilizing digital technology and improving clinical trial operations. Chugai is aiming to further accelerate the clinical trial process by improving the value chain efficiency, which is one of the basic strategies of “CHUGAI DIGITAL VISION 2030.” Chugai, NCCHE, which utilizes PhambieLINQ®, and NTT DATA conducted collaborative research to link major clinical data, including adverse events and concomitant medications, required for any clinical trials regardless of the disease to EDC.
Collaborative research
| Objective | Find solutions for technical and operational challenges when utilizing clinical data including those on adverse events and concomitant medications recorded in EMR (enhancing the efficiency of reporting/collecting case data). |
| Research content | 1. Confirm the feasibility of electronically linking clinical data, including those on adverse events and concomitant medications, from EMR in NCCHE to EDC in Chugai using PhambieLINQ® provided by NTT DATA *Included clinical data variables: adverse events, concomitant medications, patient demographics, vital signs, and clinical laboratory tests |
| Research period | From June 2023 to January 2024 |
| Verification results | 1. Electronic linkage from EMR to EDC was confirmed possible to a certain degree and possible usability in actual clinical trials was shown in terms of operability and reliability (including data quality).
(a) The number of automatic deliveries of system queries due to the EDC was reduced from 11 to 6 queries per patient. The number of manual queries was reduced from 9 to 2 queries per patient. (b) Time required for transcription in the medical institution was confirmed to be shortened by approximately 1 hour per patient.Time required for SDV work in the pharmaceutical company was confirmed to be shortened by approximately 1.5 hours per patient.Reduction of psychological burden on EDC entry in the medical institution was confirmed. |
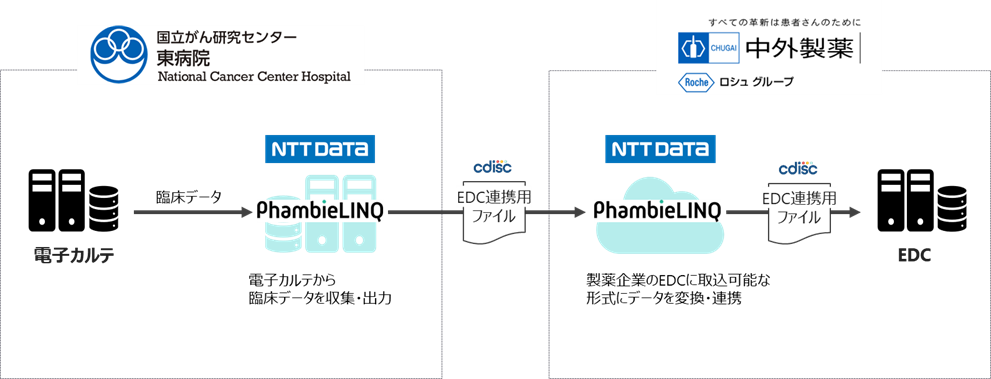
Figure: Overall image of data linkage from the medical chart to EDC in this collaborative research
Future perspective
NCCHE, Chugai, and NTT DATA will further link data including adverse events and concomitant medications in actual clinical trials based on the verification results of this research.
The advanced data linkage accomplished in this research will lead to the enhancement of efficiency and quality of clinical trial operations in the entire industry to the benefit of other medical institutions and pharmaceutical companies. To propel the preparation/popularization of the system necessary for data linkage, coordination and cooperation of whole industry is essential, including structural recording/management of clinical data including adverse events and concomitant medications at each medical institution, as well as standardization/optimization of the format of clinical data collection among the pharmaceutical companies.
To realize a world in which new drugs can reach patients as quickly as possible, NCCHE, Chugai, and NTT DATA will create a mechanism that enables efficient development and optimal quality of new drugs. We will use verified results of this research to promote this mechanism across the entire industry and enhance the efficiency of staff work to compensate for the shortage of workforce in the increasingly complicated and continuously expanding world of clinical trials.
Comments from each party
It is wonderful that the results of this collaborative research showed the possibility of improving the operations at both medical institutions and sponsors. It is important that the three parties work together to improve the clinical trial infrastructure in Japan and keep up with progress in the world. The current issue of drug lag/loss in Japan is partially attributable to the high cost of clinical trials. We believe we can lower the monitoring cost by the approach mentioned above and advance initiatives at our hospital to promote remote monitoring. On the other hand, it is debatable as to how widespread the system can become because the number of institutions that can implement the data linkage system is limited due to conditions including costs. As a clinical trial site, we will ensure the required quality while enhancing operational efficiency, and will continue to seek the optimal method to provide patients with drugs, which is our ultimate goal.
Yasutoshi Kuboki, Head of Clinical Research Management Division, Research Support Office, National Cancer Center Hospital East
Collecting clinical trial data efficiently while ensuring high quality is a common challenge for the pharmaceutical industry. We are very pleased that our accumulated knowledge and experience can be used for the effort to build a common clinical trial platform for medical institutions and pharmaceutical companies by utilizing digital technologies. Chugai is aiming to further accelerate the clinical trial process by optimizing all value chains, which is one of the basic strategies of CHUGAI DIGITAL VISION 2030. We will continue to contribute to the creation of an environment where new drugs can reach patients as quickly as possible by enhancing the efficiency and the quality of clinical trial operations.
Takao Suzuki, Associate Vice President, Head of Digital Transformation Unit, Chugai Pharmaceutical Co., Ltd.
We are very pleased to have completed the collaborative research on the world-leading theme of digitization of clinical trial operations with Chugai and NCCHE. Based on our Digital Healthcare VisionNote5, NTT DATA is accelerating our efforts to solve the social issues in the healthcare field through digitization by collaborating with aspiring customers and co-creation partners. By utilizing the knowledge obtained through this collaborative research, we will contribute to the enhancement of efficiency and quality of clinical trial operations, aiming to realize a society where everyone can receive high-quality medical services with equal quality and everyone can live a healthy long life in a safe and secure environment.
Chie Aoki, Senior Vice President, Head of Second Public Sector, Public Headquarters, NTT DATA Japan.
Glossary
(Note1) EDC (electronic data capture system)
EDC is a mechanism or its system to collect clinical data electronically.
(Note2) PhambieLINQ®
“PhambieLINQ®” is a clinical trial integration platform developed by NTT DATA to link medical institutions and pharmaceutical companies. It enables data linkage between medical institutions and pharmaceutical companies, and contributes to the enhancement of efficiency of clinical trial processes. “PhambieLINQ®” is a trademark of NTT DATA Japan. in Japan. https://phambielinq.com/(linked at external site)
(Note3) Source: Japan Pharmaceutical Manufacturers Association. “Drug Information Q & A”
https://www.jpma.or.jp/about_medicine/guide/med_qa/q33.html (linked at external site)(Accessed May, 2024) (Japanese only)
(Note4) SDV (Source Data Verification)
SDV refers to the operation of verifying ethical and scientific appropriateness of the conduct of clinical trials and the reliability of data.
(Note5) NTT DATA’s Digital Healthcare Vision
NTT DATA has published a white paper on digital healthcare that depicts “Harmonized Well-being with Data” https://www.nttdata.com/jp/ja/industries/healthcare/ (linked at external site)(Accessed May 2024)
*Product names, company names, and association names are the trademarks or registered trademarks of their respective companies.
Contact information
National Cancer Center (Japan)
Contact from the media
Office of Public Relations, Strategic Planning Bureau
E-mail: ncc-admin●ncc.go.jp
Chugai Pharmaceutical Co., Ltd.
Contact from the media
Media Relations Group, Corporate Communications Dept.
Tel: +81-3-3273-0881
E-mail: pr●chugai-pharm.co.jp
Contact from investors
Investor Relations Group, Corporate Communications Dept.
Tel: +81-3-3273-0554
E-mail: ir●chugai-pharm.co.jp
NTTDATA Japan.
Contact from the media
Public Relations Department,
E-mail: nttdata-pr-inquiries●am.nttdata.co.jp
Related to this collaborative research and solutions
Hoshino, Digital Welfare Department, Second Public Sector
E-mail: phambielinq-sales●am.nttdata.co.jp
About National Cancer Center Hospital East
At National Cancer Center Hospital East, we provide our patients with world-class cancer treatments, multidisciplinary teams consisting of various specialities coordinate the best support for each patient, delivered with utmost care. We offer advanced medical services using state-of-the art medical devices such as robot-assisted surgery, proton beam and endoscopic therapy, minimally invasive surgery limiting burdens to the patient. We also provide various drug treatments and conduct many clinical trials.
URL: https://www.ncc.go.jp/jp/ncce/index.html
About Chugai
Chugai Pharmaceutical Co., Ltd., headquartered in Tokyo, is a research-based pharmaceutical company with world-class drug discovery capabilities, including proprietary antibody engineering technologies. Chugai is committed to creating innovative pharmaceutical products that may satisfy unmet medical needs. Chugai is listed on the Prime Market of the Tokyo Stock Exchange. While maintaining autonomy and management independence, Chugai is an important member of the Roche Group. Additional information is available at https://www.chugai-pharm.co.jp/english/(linked at external site).
About NTT DATA
NTT DATA - a part of NTT Group - is a trusted global innovator of IT and business services headquartered in Tokyo. We help clients transform through consulting, industry solutions, business process services, IT modernization and managed services. NTT DATA enables clients, as well as society, to move confidently into the digital future. We are committed to our clients' long-term success and combine global reach with local client attention to serve them in over 50 countries. Visit us at nttdata.com.
