Home > Information > press release > Development of a Novel Nucleic Acid Drug Discovered by National Cancer Center
Development of a Novel Nucleic Acid Drug Discovered by National Cancer Center
July 7, 2015
National Cancer Center
in Japanese
National Cancer Center (NCC; President: Tomomitsu Hotta, Chuo-ku, Tokyo) hereby announces that a novel siRNA nucleic acid drug formulation “TDM-812”, which suppresses Ribophorin II (RPN2) gene expression that regulates the treatment-resistibility of breast cancer and discovered by NCC Research Institute (Director: Hitoshi Nakagama), has been developed in collaboration with 3-D Matrix, Ltd (President: Kentaro Takamura, Chiyoda-ku, Tokyo). An investigator-initiated clinical trial (first-in-human Phase I study) has been started at NCC Hospital (Director: Yasuaki Arai) and TDM-812 was successfully administered to the first subject.
As nucleic acid drug acts to suppress abnormal genes, it has fewer side effects and it can be expected to treat the root cause of the disease. However, so far none has been approved as anticancer drug due to difficulties in stabilization and efficient delivery of the drug into cancer cells in vivo. TDM-812 has resolved these problems through its formulation, and efficacy has already been proven in a pre-clinical study of large animal models. Starting with this investigator-initiated clinical trial, we will work towards the first nucleic acid drug for treatment of breast cancer in the world.
In treatment-resistant locally advanced or recurrent breast cancer, large mass or skin ulcers (with partial loss of skin) commonly form when lesions progress to the skin around the primary tumor or surrounding lymph nodes. Local pain, bleeding, malodor, and exudate are also often experienced, which affects the patient’s Quality of Life (QOL). It is hard to control local lesions with existing therapies, hence development of a new treatment is sort after to improve patient’s QOL.
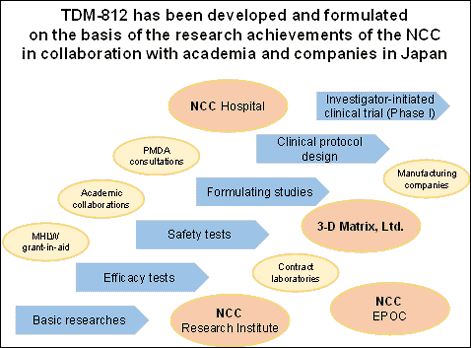
TDM-812 has been developed and formulated on the basis of the research achievements of the National Cancer Center in collaboration with a Japanese company, 3-D Matrix, Ltd (3-D Matrix). This investigator-initiated clinical trial is the first study for patients with treatment-resistant locally advanced or recurrent breast cancer in the world.
RPN2 gene in breast cancers
The function of the targeted gene RPN2 has been discovered by the Division of Molecular and Cellular Medicine, NCC Research Institute (Chief: Takahiro Ochiya), and findings have been published on Nature Medicine in 2008 (Nat. Med., 14: 939-948, 2008). They have also found that RPN2 gene controls “cancer stem cells”, which produce new cancer cells, and also regulate the treatment-resistibility. Furthermore, by applying RNA-interference (RNAi), it has been proven that knockdown of expression of RPN2 by introducing siRNA nucleic acid suppresses the nature and proliferation of drug-resistant breast cancer cells.Nucleic acid drug
As nucleic acid drug acts to suppress abnormal genes, it has fewer side effects and it can be expected to treat the root cause of the disease. In spite of many researches on a variety of genes, none has been approved as anticancer drug so far.Formulation of RPN2siRNA
Being easily degradable in vivo, siRNA and other nucleic acid drugs in naked form is hard to be delivered into cells, resulting in little efficacy. To efficiently deliver siRNA into cancer cells while avoiding degradation, it is necessary to develop a suitable drug formulation. NCC and 3-D Matrix have jointly developed TDM-812, which is a complex drug formulation consisting of RPN2siRNA and surfactant-like peptide A6K. A6K has been developed by 3-D Matrix with possibility of solving problems of degradation and drug delivery into cells by forming a complex with RPN2siRNA. Efficacy of TDM-812 has already been proven in a pre-clinical study in large animal models with breast cancers. The treatment with TDM-812 is expected to be a highly specific therapy for cancer cells because RPN2 is localized in cancer stem cells and almost no expression of RPN2 is seen in normal tissues.Treatment-resistant breast cancer
Breast cancer is the most common cancer in Japanese women and cases are predicted to increase drastically. Chemotherapies such as hormone therapy or anticancer drugs are commonly administered to treatment-resistant metastatic or recurrent breast cancer patients.Investigator-initiated clinical trial for treatment-resistant local mass
This investigator-initiated clinical trial is a first-in-human Phase I study, intended for patients suffering from treatment-resistant breast cancer with local mass. The study has been conducted at the Department of Breast and Medical Oncology of the National Cancer Center Hospital (Department Chief: Kenji Tamura). Safety and tolerability of intratumorally administered TDM-812 will be evaluated, and the recommended dose of intratumoral administration is aimed to be determined.This is the first time in Japan where discovery of a particular gene led to formulation of a breast cancer treatment nucleic acid drug for clinical trial. On June 30th 2015, a patient with triple-negative breast cancer with subcutaneous metastasis has been treated with TDM-812 as the first subject of this investigator-initiated clinical trial.
Contact info
Regarding Investigator-initiated clinical trialResearch Management Section, Research Coordination Division, Center for Research Administration and Support
National Cancer Center
5-1-1, Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
Phone: 81-3-3542-2511 (Ext 5661)
E-mail: RPN2-office[at]ml.res.ncc.go.jp (Please replace [at] to @.)
Regarding Surfactant peptide A6K
3-D Matrix, Ltd.
3-2-4, Kojimachi, Chiyoda-ku, Tokyo 102-0083, Japan
Phone 81-3-3511-3440
URL: http://www.puramatrix.com
Inquiry from media
Office of Public Relations, Strategic Planning Bureau
National Cancer Center
5-1-1, Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
Phone: 81-3-3542-2511
Fax 81-3-3542-2545
E-mail: ncc-admin[at]ncc.go.jp (Please replace [at] to @.)