Home > Information > press release > Malignant extracellular vesicles derived from ovarian cancer cells facilitate peritoneal dissemination, and the vesicles can be promising targets for improving patient outcomes.
Malignant extracellular vesicles derived from ovarian cancer cells facilitate peritoneal dissemination, and the vesicles can be promising targets for improving patient outcomes.
February 28, 2017
National Cancer Center
Nagoya University
Japan Agency for Medical Research and Development
in Japanese
Ovarian cancer is the most lethal gynecological malignancy, and the lethality mainly attribute to the frequency of peritoneal metastasis. Peritoneal dissemination of cancer cells is responsible for the high morbidity and mortality not only of ovarian cancer but also of gastrointestinal cancer. Unfortunately, the prognosis of cancer patients with peritoneal dissemination has not improved over the last several decades, and furthermore, very little is known about the molecular mechanisms underlying this type of metastasis.
Almost all cell types, including tumor cells, secrete EVs, and tumor-derived EVs play an important role in cancer initiation and progression by carrying various bioactive molecules. Over the last several years, the biological functions of EVs related to tumor metastasis have been investigated, and here, we identified for the first time the EVs involved in the process of peritoneal dissemination.
It was clearly demonstrated that EVs derived from high metastatic ovarian cancer cells, ES-2 cells, promote peritoneal dissemination in vivo. Next, it was found that the mesothelial cells which are main component of peritoneal membrane, were structurally broken by treatment with the EVs from ES-2 cells in vitro and in vivo because of inducing apoptosis by the malignant EVs. From microarray gene expression analysis, MMP1 gene was significantly upregulated in mesothelial cells which treated with malignant EVs. Thus, MMP1 was selected and validated by qRT-PCR at high reproducibility. Interestingly, MMP1 mRNAs also packaged in the EVs from ES2, and it was confirmed that the gene was a key molecule for the destructive phenotypes. MMP1 was confirmed as a strong prognostic factor for early ovarian cancer patients according to open access database. In addition, the EVs which contained MMP1 mRNAs also existed in the EVs derived from ovarian cancer patients’ ascites and furthermore the EVs also induced apoptosis in vitro.
The current study identified EV-related mechanisms of peritoneal dissemination. The evidence for the involvement of EVs is clearly demonstrated by in vitro and in vivo experiments, and the existence of malignant EVs, which contain MMP1 mRNAs and facilitate peritoneal dissemination, was verified in clinical samples. The malignant EVs may be a strong prognostic biomarker because MMP1 mRNA is significantly correlated with prognosis in stage l ovarian cancer patients. The detection of the malignant EVs can contribute to early diagnosis, prediction of metastasis and that of recurrence. The fact that MMP1 mRNA is packaged in EVs will contribute to drug discovery research because the research for MMPs is slightly stagnant due to the many previous disappointing clinical trial results by directly inhibiting MMPs. We believe that our findings will suggest alternative therapeutic strategies for MMPs. If the technology for inhibition of the EVs is developed in the near future, we will be able to manage the extracellular MMPs in EVs by indirect methods. Additionally, the blockade of the malignant EVs may prevent metastases. Thus, a newly identified malignant EVs may be promising targets for improving patient outcomes.
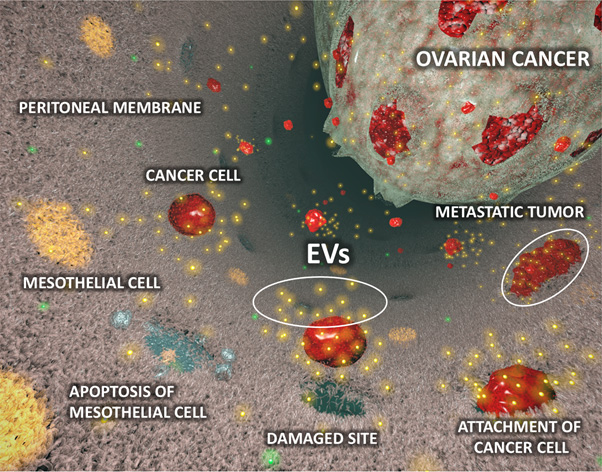
Figure: The diagram of peritoneal dissemination involved in extracellular vesicles (EVs) derived from ovarian cancer cells
Publication
Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancerAkira Yokoi, Yusuke Yoshioka, Yusuke Yamamoto, Mitsuya Ishikawa, Syunichi Ikeda, Tomoyasu Kato, Tohru Kiyono, Fumitaka Takeshita, Hiroaki Kajiyama, Fumitaka Kikkawa and Takahiro Ochiya Nature Communications 2017
Inquiries
National Cancer Center (NCC)
E-mail: ncc-admin[at]ncc.go.jp (Please replace [at] to @.)
Tel: +81-3-3542-2511
Nagoya University
E-mail: iga-sous[at]adm.nagoya-u.ac.jp (Please replace [at] to @.)
Tel: +81-52-744-2228
Japan Agency for Medical Research and Development (AMED)
E-mail: cancer[at]amed.go.jp (Please replace [at] to @.)
Tel: +81-3-6870-2221
