Home > Information > press release > Precise cancer risk estimation based upon measurement of mutations and aberrant DNA methylation accumulated in normal tissues
association between the accumulation and life style
Precise cancer risk estimation based upon measurement of mutations and aberrant DNA methylation accumulated in normal tissues
association between the accumulation and life style
January 23, 2018
National Cancer Center of Japan (NCC, Japan)
Japan Agency for Medical Research and Development (AMED)
in Japanese
Essentials
- A novel method to measure rare mutations accumulated in normal tissues was developed.
- In the esophagus, both point mutations and aberrant DNA methylation increased according to the risk of esophageal squamous cell cancer. In the stomach, only aberrant DNA methylation increased according to gastric cancer risk.
- The increase was associated with smoking, drinking, and betel nut consumption in esophageal mucosa, and with Helicobacter pylori infection in gastric mucosa.
- A combination of mutations and DNA methylation is expected to provide novel precision cancer risk diagnosis, reflecting individuals' life history.
A research group at the National Cancer Center of Japan has developed a new method that can measure rare point mutations accumulated in normal tissues. The research group further measured point mutations and aberrant DNA methylation levels in normal esophageal and gastric mucosa with different levels of cancer risk.
The group found that accumulation of both mutations and aberrant DNA methylation was associated with esophageal squamous cell cancer (ESCC) risk, and accumulation of methylation only with gastric cancer risk. The accumulation reflected lifestyles: smoking, drinking, and betel nut consumption in esophageal mucosa, and Helicobacter pylori (H. pylori) infection in gastric mucosa. A combination of mutations and methylation provided precise cancer risk estimation.
Risk prediction using normal tissue before a cancer develops leads to personalized cancer screening, and potentially prevention. The same strategy can be applied to other organs and lifestyle risk factors. The researchers are now conducting a multicenter prospective study to predict gastric cancer risk in healthy individuals who received H. pylori eradication.
The study was conducted by a research group in Division of Epigenomics (Chief Toshikazu Ushijima). The study was supported by research funds from the AMED and NCC, and was published in Proceeding of National Academy of Sciences.
What are DNA methylation and point mutations?
DNA methylation is a mark in the genome for a cell to remember what genes are to be used and what are not. Its abnormality (aberrant DNA methylation) can permanently inactivate a gene without altering genetic information coded in DNA sequence. Aberrant DNA methylation is known to be induced by chronic inflammation, such as gastritis due to H. pylori infection. The research group previously demonstrated that aberrant DNA methylation levels in gastric mucosa are closely correlated with gastric cancer risk.
Mutations are alterations in genetic information due to errors in DNA sequence. They are induced by mutagens, such as tobacco, fungal toxins, heterocyclic amines, and radiation, and are permanent. However, their accumulation levels in normal tissues are so low that their measurement has been extremely difficult. The research group developed a novel method to measure low levels of point mutations accumulated in normal tissues.
Background
The vast majority of adult cancers develop as a result of accumulation of mutations and aberrant DNA methylation due to aging and lifestyles, such as smoking, drinking, and H. pylori infection. The mutations and aberrant DNA methylation are accumulated in normal tissues far before a cancer develops. Since their accumulation levels are very low, their measurement has been extremely difficult.
However, regarding aberrant DNA methylation, the research group identified appropriate marker genes, and showed that accumulation levels of DNA methylation in gastric mucosa were correlated with gastric cancer risk in 2006. They started a multicenter prospective study to predict a risk of a next gastric cancer in gastric cancer patients treated with endoscopy in 2008, and finally demonstrated in 2017 that high accumulation levels of aberrant DNA methylation increase the risk three-fold (Maeda et al, Gut, 66:1721, 2017).
On the other hand, it has been difficult to measure rare point mutations, and the research group has been trying to overcome this issue. In 2017, they introduced a simple invention into preparation of samples for next-generation sequencers, and overcome the low fidelity of sequencers to detect rare point mutations. The method successfully measured point mutations at as low as one point mutation per 105 base pairs (Yamashita et al, Cancer Lett, 403:152, 2017).
Previous press release
Dec 21, 2016 "New Risk Diagnosis Technique for Additional Gastric Cancer After Endoscopic Curative Treatment of A Gastric Cancer"
https://www.ncc.go.jp/en/information/press_release/20161221/index.html
Research Methods
The research group collected esophageal and gastric mucosa biopsy samples from individuals with three different cancer risk levels, and measured point mutations and aberrant DNA methylation in the samples.
- Low-risk individuals
People who have not exposed to lifestyle risk factors.
- Esophagus: 30 people (No history of smoking, drinking, or betel nut; 20 males and 10 females)
- Stomach: 32 people (No history of H. pylori infection; 18 males and 14 females) - Intermediate-risk individuals
People exposed to lifestyle risk factors, but who have not developed cancer.
- Esophagus: 32 people (with history of smoking, drinking, or betel nut but without cancer; 32 males)
- Stomach: 32 people (with history of H. pylori infection but without cancer; 22 males and 10 females) - High-risk individuals:
People exposed to lifestyle risk factors, and who have developed cancer.
- Esophagus: 31 people (with history of smoking, drinking, or betel nut and with cancer; 31 males)
- Stomach: 32 people (with history of H. pylori infection and gastric cancer; 25 males and 7 females)
Research Results
1. Point mutations and/or aberrant DNA methylation increased according to cancer risk
In the esophagus, both point mutations and aberrant DNA methylation increased according to ESCC risk. In contrast, in the stomach, only DNA methylation increased according to gastric cancer risk (Fig. 1).
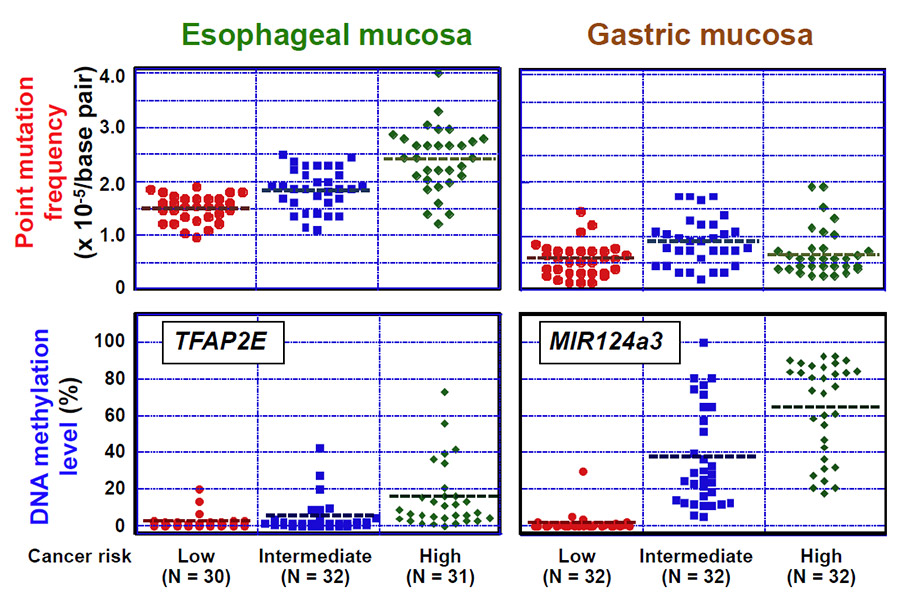
Fig. 1 Correlation between point mutations/aberrant DNA methylation and cancer risk
2. Increased point mutations and DNA methylation levels reflect lifestyles.
For ESCCs, smoking, drinking, and betel nut consumption, especially in Taiwan, have been established as risk factors. Smoking and drinking induce both point mutations and aberrant DNA methylation, which coincided with the results here. For gastric cancers, H. pylori infection has been established as a strong risk factor. H. pylori infection is known to potently induce aberrant DNA methylation, which again coincided with the results here (Fig. 2).
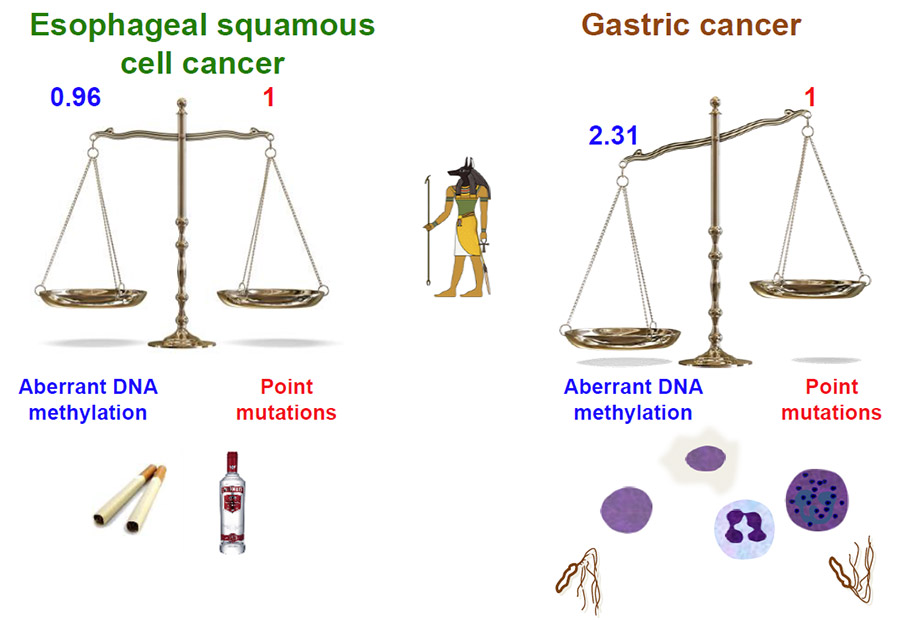
Fig. 2 Lifestyle risk factors and their roles in the relative importance of point mutations and aberrant DNA methylation
3. Toward precision cancer risk diagnosis based upon a combination of point mutations and aberrant DNA methylation
Until now, we were able to measure only aberrant DNA methylation in normal tissues. Now, by combining with point mutations, risk estimation is expected to become more precise. In the case of ESCCs, such combination indeed improved risk estimation. In the case of gastric cancer, prediction power of aberrant DNA methylation is sufficiently high, and the usefulness of addition of point mutations was unclear (Table 1).
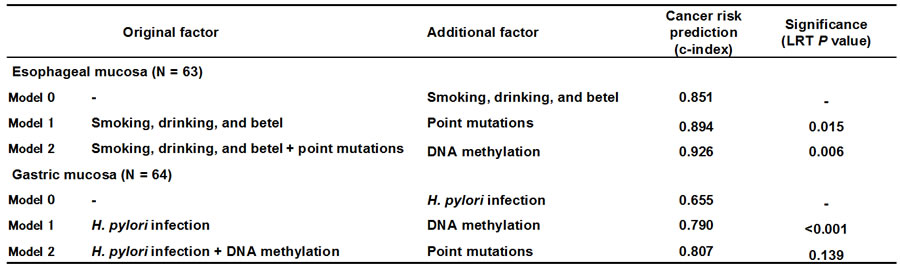
Table 1 Prediction power of cancer risk by addition of point mutations and aberrant DNA methylation on top of lifestyle risk factors
C-index of 1 means 100% sensitivity and specificity.
To prevent ESCCs, suppression of both point mutations and aberrant DNA methylation by quitting smoking and reducing drinking is important. To prevent gastric cancers, suppression of aberrant DNA methylation by preventing and eradicating H. pylori is important.
A multicenter prospective study to predict gastric cancer risk in healthy individuals
The research group previously confirmed that methylation levels in gastric mucosa are closely associated with cancer risk. To bring the finding to our society, the group is conducting a multicenter prospective study to predict gastric cancer risk by accumulation level of aberrant DNA methylation in gastric mucosa in healthy individuals who received H. pylori eradication. The study involves 67 institutions nation-wide, and has recruited 1,479 individuals with a target number of 2,000.
Perspectives
The results here demonstrated that measurement of both point mutations and aberrant DNA methylation in normal tissues has a promise as precision cancer risk diagnosis. Acceleration of the ongoing multicenter prospective study will bring the finding to practice earlier. Analysis of further variety of cancers will illuminate our cancer etiology and their association with point mutations and aberrant DNA methylation.
Paper
- Journal
Proceeding of National Academy of Sciences - Title
Genetic and epigenetic alterations in normal tissues have differential impacts on cancer risk among tissues. - Authors
Satoshi Yamashita, Takayoshi Kishino, Takamasa Takahashi, Taichi Shimazu,Hadrien Charvat, Yasuo Kakugawa, Takeshi Nakajima, Yi-Chia Lee, Naoko Iida, Masahiro Maeda, Naoko Hattori, Hideyuki Takeshima, Reiko Nagano, Ichiro Oda, Shoichiro Tsugane, Ming-Shiang Wu, and Toshikazu Ushijima - DOI
10.1073/pnas.1717340115
Research Fund
- The Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED) (17ck0106267h0001)
- The Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT) from the Japan Agency for Medical Research and Development (AMED) (17cm0106517h0002)
- The National Cancer Center Research and Development Fund (H29-E-7)
Inquiries
From Media
- Office of Public Communication, National Cancer Center
Phone: +81-3-3542-2511 E-mail:ncc-admin●ncc.go.jp(Please replace ● with @) - The Japan Agency for Medical Research and Development (AMED)
Phone: +81-3-6870-2221 E-mail:cancer●amed.go.jp(Please replace ● with @)
From Scientists
Dr. Toshikazu Ushijima, National Cancer Center Research Institute
Phone: +81-3-3542-2511 E-mail:tushijim●ncc.go.jp(Please replace ● with @)
