Home > Information > press release > SCRUM-Japan GI-SCREEN Aims for Realizing of Cancer Precision Medicine Utilizing Liquid Biopsy by Analyzing Comprehensive Cancer Genome Alterations in Blood
SCRUM-Japan GI-SCREEN Aims for Realizing of Cancer Precision Medicine Utilizing Liquid Biopsy by Analyzing Comprehensive Cancer Genome Alterations in Blood
March 13, 2018
National Cancer Center, Japan
in Japanese
In February 2018, National Cancer Center (President, Dr. Hitoshi Nakagama, Tokyo, Japan) and National Cancer Center Hospital East(Director, Dr. Atsushi Ohtsu, Kashiwa, Japan) launched a new project “Research on Liquid Biopsy in Patients with Advanced Gastrointestinal Cancers”. This study is conducted using a highly sensitive genetic analysis technology “Guardant360(R) assay” as part of a Nationwide cancer genome screening project for various gastrointestinal cancer, “SCRUM-Japan GI-SCREEN”. The Guardant360(R) assay, developed by Guardant Health in the U.S. is a new diagnostic technique capable of analyzing fragments of tumor DNA circulating in the blood by next-generation sequencer technology, and providing cancer genetic information accurately and quickly. As conventional tumor tissue biopsies are highly invasive, biopsies of multiple regions and repeated biopsies can cause significant risk to the patients and delays in reaching a treatment decision. However, liquid biopsy is minimally invasive and enables the analysis of fragments of tumor DNA circulating in the blood. For these reasons, it can overcome the problems faced by tumor tissue biopsy.
Background/social significance
Background/social significanceVarious genetic alterations have been being discovered in gastrointestinal cancers with the progress of genetic analysis; findings are applicable to clinical treatments. For instance, anti-epidermal growth factor receptor (anit-EGFR) antibodies such as cetuximab and panitumumab are used for treating colorectal cancers, but they are ineffective if the RAS gene has a mutation. Thus, the RAS gene test is now carried out before starting the anti-EGFR antibody therapy. However, alterations of various genes other than RAS have been reported to be related with resistance to anti-EGFR antibodies, such as BRAF, PIK3CA, HER2, and MET genes. At the moment, it is not clear whether anti-EGFR antibody preparations are completely ineffective in clinical treatment if these genetic alterations are found. Thus, the testing of these genes is presently not covered by National Health Insurance. For other gastrointestinal cancers, such as gastric, esophageal, liver, biliary tract, pancreatic, small intestine, appendicitis, anal canal cancers, gastrointestinal neuroendocrine tumor/cancer, and gastrointestinal stromal tumor (GIST), the only treatment option based on genetic alterations available in the clinical scene is HER2 gene amplification for gastric cancer. Presently, new drugs for digestive system cancers with these genetic alterations are being developed, thus the importance of the analysis of these genetic alterations is gradually being recognized.
These genetic alterations have been gradually known to change according to the tumor region and treatment effects. Conventional tumor tissue biopsies are highly invasive and impose considerable stress on patients,
making it difficult to analyze genes by the biopsies of multiple regions and repeated biopsies. On the other hand, fragments of tumor DNA are known to circulate in the blood, allowing analysis by liquid biopsy using sampled blood. It can overcome the problems faced by tumor tissue biopsy. If the usefulness of genetic analysis using minimally invasive liquid biopsy for gastrointestinal cancers can be confirmed, even more precise personalized medicine based on genetic analysis using liquid biopsy will be established soon.
Outline of study
The joint industry-academia Nationwide cancer genome screening project “SCRUM-Japan GI-SCREEN” is a genetic alteration screening project for patients with gastrointestinal cancers, undertaken by the National Cancer Center Hospital East in collaboration with medical facilities all over the country and pharmaceutical companies, in the aim to provide the best medical care to each and every patient. If specific genetic alterations are found in patients in this study, they may participate in clinical studies of corresponding treatment drugs, which gives opportunities for new treatments. As of December 2017, more than 5,000 patients with gastrointestinal cancers have been registered since 2014.
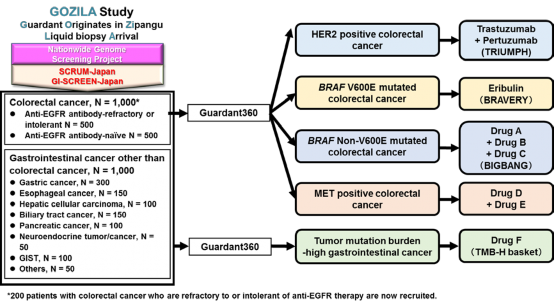
As part of SCRUM-Japan GI-SCREEN, a new Project “Research on Liquid Biopsy in Patients with Advanced Gastrointestinal Cancers” will start. The project will adopt Guardant360(R) assay, a new genetic analysis technology capable of measuring alterations in 73 genes all at once from blood. To date, cancer tissue specimens has been used for genetic analysis, but this study will attempt at genetic analysis using the blood (20 mL) of patients with gastrointestinal cancers. Specimens will be sent to Guardant Health who will conduct the genetic analysis to determine if the 73 cancer-related genes such as RAS, BRAF, PIK3CA, HER2, and MET are abnormal. The results of the genetic analysis will take about two weeks. The study will first be conducted on about 200 patients with advanced colorectal cancer who underwent treatment with anti-EGFR antibody. In upcoming year, the study will be expanded to appropriately 2,000 patients with all advanced gastrointestinal cancers to verify the clinical utility of genetic analysis using liquid biopsies. If specific genetic alterations are found in patients in this study, they may participate in clinical studies of corresponding treatment drugs.
Figure 1. List of genetic alterations that can be analyzed by Guardant360(R) assay
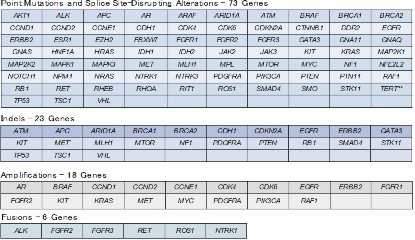
Figure 2. Outline of study
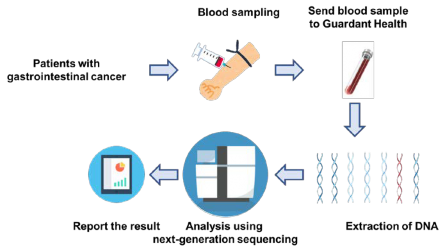
Figure 3. Future plan
Prospects
If the study results can identify different genetic alterations linked to cancer treatment in the blood and the differences from genetic alterations in the tumor tissue, it will help understand the changes in genetic alterations linked to cancer, as well as enable reviews for realizing of cancer precision medicine using liquid biopsies.
SCRUM-Japan GI-SCREEN
SCRUM-Japan GI-SCREEN (Principal investigator, Dr. Takayuki Yoshino, Director of the Department of Gastroenterology and Gastrointestinal Oncology in National Cancer Center Hospital East) is the Nationwide cancer genome screening project for patients with advanced gastrointestinal cancers, undertaken by the National Cancer Center Hospital East in collaboration with medical facilities all over the country and pharmaceutical companies. It was established to identify cancer patients with orphan-fractionated cancer genome alteration who likely to have promising therapeutic drugs and to prepare easy-to-access platform where the patients can receive new treatment, in collaboration with major cancer hospitals and universities in Japan since February 2014. At the time of the start, it aimed to find orphan-fractionated advanced colorectal cancer, but since February 2015, it became a member of the joint industry-academia nationwide cancer genome screening project “SCRUM-Japan”, which expanded to not only colorectal cancer but also the whole gastrointestinal cancer, such as gastric and esophageal cancer.
Contact
Contact from patients
National Cancer Center (Kashiwa Campus)
SCRUM-Japan Secretariat
E-mail address: scrum_office●east.ncc.go.jp(replace ● to @)
Contact from the press
National Cancer Center
Office of Public Relations, Strategic Planning Bureau (Kashiwa Campus)
+81-4-7133-1111 (main), +81-4-7134-6945 (direct line)
E-mail address: ncc-admin●ncc.go.jp(replace ● to @)
