Home > Information > press release > Cancer Gene Panel Test OncoGuideTM NCC Oncopanel System added to Health Insurance Coverage list
Cancer Gene Panel Test OncoGuideTM NCC Oncopanel System added to Health Insurance Coverage list
May 29, 2019
National Cancer Center
in Japanese
OncoGuideTM NCC Oncopanel System (hereinafter, “NCC Oncopanel Test”) has been listed for coverage under national health insurance from June 2019. NCC Oncopanel Test was developed jointly by the National Cancer Center (President: Hitoshi Nakagama, Location: Chuo-ku, Tokyo, Japan) and Sysmex Corporation (Chairman and CEO: Hisashi Ietsugu; HQ: Kobe, Japan) as a type of gene panel testing*1 designed specifically for Japanese cancer genome mutations.
The NCC Oncopanel Test uses next-generation sequencers*2 to test 114 genes where Japanese people are prone to express cancer mutations, in a single round. Comprehensive examination of gene mutations causing various types of solid tumors including childhood cancers, aids in patient diagnosis, in therapeutic drugs selection and realizes cancer genomic medicine*3.
Comment from Hitoshi Nakagama, President - National Cancer Center
“Insurance coverage of cancer genomic medicine, allowing optimal treatments for individuals based on cancer genome mutation information is groundbreaking. The Center for Cancer Genomics and Advanced Therapeutics (C-CAT)*4 will aggregate the generated cancer genome information with clinical details. A framework to make the most out of the available information, sharing with academia, pharmaceutical and medical devices companies, while safeguarding data protection, is being put together. With nationwide collaboration, we shall take cancer genomic medicine in Japan to the global forefront.”
Clinical Utility
NCC Oncopanel Test detects mutations in 114 genes, evaluates the tumor mutation burden*6 affecting treatment effects of immune checkpoint inhibitors, while differentiating mutations cancer patients are born with (germline mutations*7) and mutations present only in cancer cells (somatic gene mutations). Consequently, in some cases test results can help diagnose hereditary cancers.
At TOP-GEAR Project*8study at the National Cancer Center Hospital, which verifyied this test, gene mutations than can guide therapeutic decisions*9 were detected in approximately half the patients. Anti-cancer drugs were administered to more than 10% of patients based on the gene mutations discovered. In fiscal 2018, more than 300 patients received testing as advanced medical care, confirming its operability and utility.
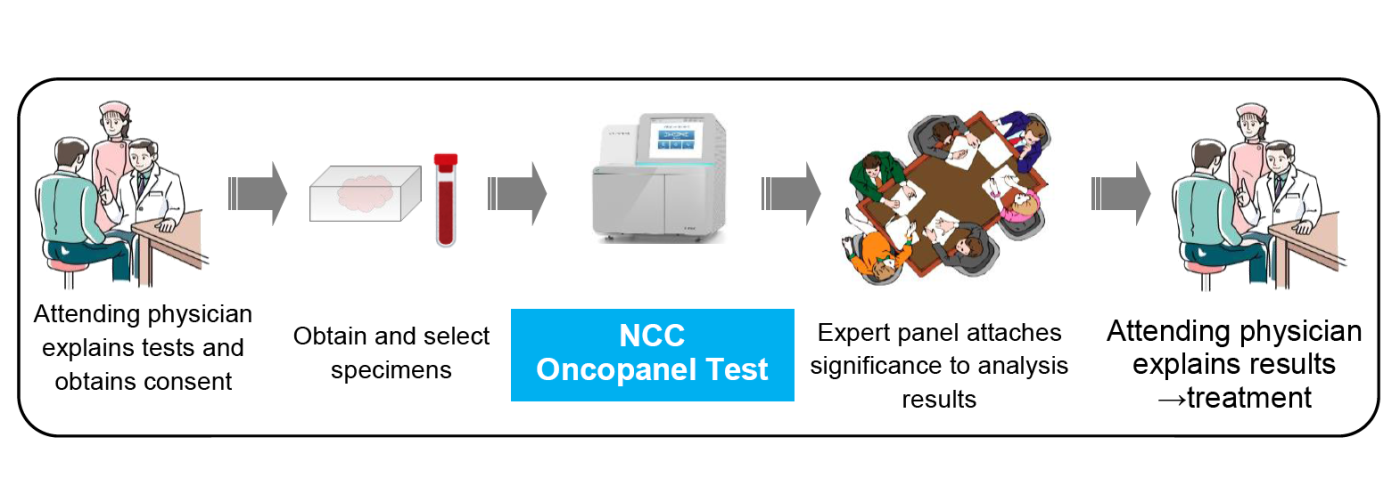
figure : NCC Oncopanel Test procedure
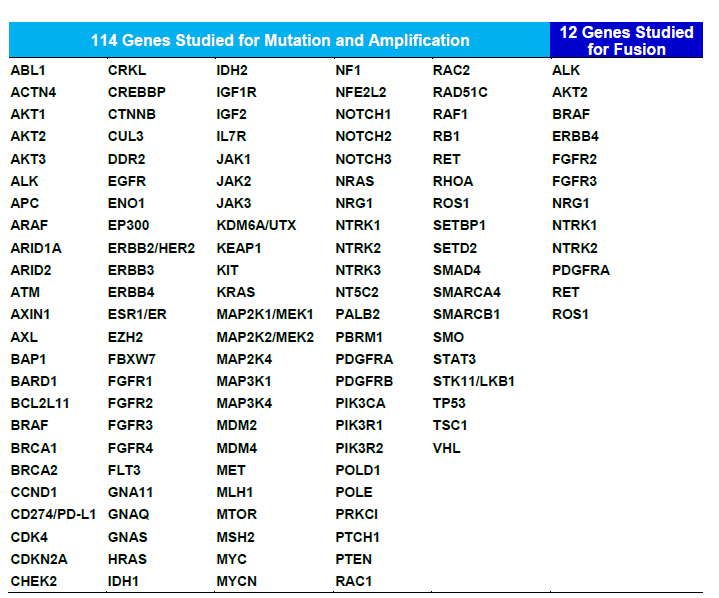
table : Genes studied in the NCC Oncopanel Test
Reference
Please see the following for details on insurance coverage.
Central Social Insurance Medical Council (General Assembly of the Central Social Insurance Medical Council) - Japanese only
https://www.mhlw.go.jp/stf/shingi/shingi-chuo_128154.html
Research Supported by
Japan Agency for Medical Research and Development (AMED)
Program for an integrated database of clinical and genomic information
Pointing toward genomic drug discovery and treatment of advanced solid tumors on a nationwide scale and creating storage for clinical genomic data on hereditary tumors (JP18kk0205004)
Project for the practical realization of innovative medical seeds
Research toward the practical realization of cancer gene profiling (JP19lk1403003)
National Cancer Center R&D Funds
Creating a foundation for the development of personalized medicine using gene mutation and other information (24-A-1)」
Research related to preparing the foundations for clinical sequencing for use in personalized medicine (27-A-1)
Research related to putting in place a system for clinical sequencing to contribute to personalized cancer medicine in Japan (30-A-6)
Terminology
Testing that studies cancer-related mutations in numerous genes at once. Also referred to as gene profiling. This type of testing burdens patients less, eliminating the need for repeated biopsies, and in time, compared to multiple individual gene mutations tests.
*2 Next-generation sequencers:
Analyzers for reading huge volumes of information contained in DNA bases and sequences. This test used the NextSeq 550Dx (class I medical device, manufacturing and sales notification number: 13B2X10271000001), manufactured by Illumina.
The treatment of cancer based on information on genetic mutations. More highly effective therapeutic drugs can be selected based on genetic mutations, facilitating “personalized medicine,” tailored to individual patients.
https://ganjoho.jp/public/dia_tre/treatment/genomic_medicine/genmed02.html
*4 Center for Cancer Genomics and Advanced Therapeutics (C-CAT):
Established by the Japanese government within the National Cancer Center on June 1, 2018. The center was found as a hub for aggregating and managing nationwide information on cancer genomic medicine, as well for utilizing this information to enhance the quality of treatment covered by health insurance and developing new medical treatments.
https://www.ncc.go.jp/en/c_cat/index.html
Councils where specialists representing various disciplines collectively examine analysis results, interpret, and propose treatments. Members include the attending physician, an oncologist, a doctor in charge of clinical testing, a pathologist, a genome specialist, a doctor specializing in clinical genetics and a genetic counselor. Based on the panel’s deliberations, the attending physician provides the patient with explanations on gene mutations and treatment options.
*6 Tumor mutation burden (TMB):
The quantity of mutated genes within a tumor, increasingly focused on as a marker for predicting the efficacy of immune checkpoint inhibitors. Ten mutations per one million DNA bases is considered high.
Genetic mutations individuals are born with, which can indicate predispositions towards cancer.
https://www.ncc.go.jp/jp/ncch/genome/top-gear-project/index.html
Research findings are reported in the following academic paper.
Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 2019, 110(4):1480-1490. (Open Access)
*9 Gene mutations related to therapeutic decisions:
Mutations that provide useful information to the selection of therapeutic drugs, as well as in cancer diagnosis and prognosis. Diagnostic guidance (version 1.0) based on gene panel testing using next-generation sequencers is provided jointly by the Japanese Society of Medical Oncology, the Japan Society of Clinical Oncology and the Japanese Cancer Society in detail.
https://www.jsmo.or.jp/about/doc/20171011_01.pdf
Media Inquiries
Office of Public Relations, Strategic Planning Bureau, National Cancer Center
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
Phone: +81-3-3542-2511 Fax: +81-3-3542-2545
E-mail: ncc-admin●ncc.go.jp(Please replace ● with @)
