Home > Information > press release > Chugai, NCCH, OMPU and MICIN Start Company-Sponsored Phase I Study in Oncology with a New Decentralized Clinical Trial Structure
Chugai, NCCH, OMPU and MICIN Start Company-Sponsored Phase I Study in Oncology with a New Decentralized Clinical Trial Structure- Improving access to the clinical trial for patients far from the institution -
Chugai Pharmaceutical Co., Ltd.
National Cancer Center
Osaka Medical and Pharmaceutical University
MICIN
In Japanese
Overview
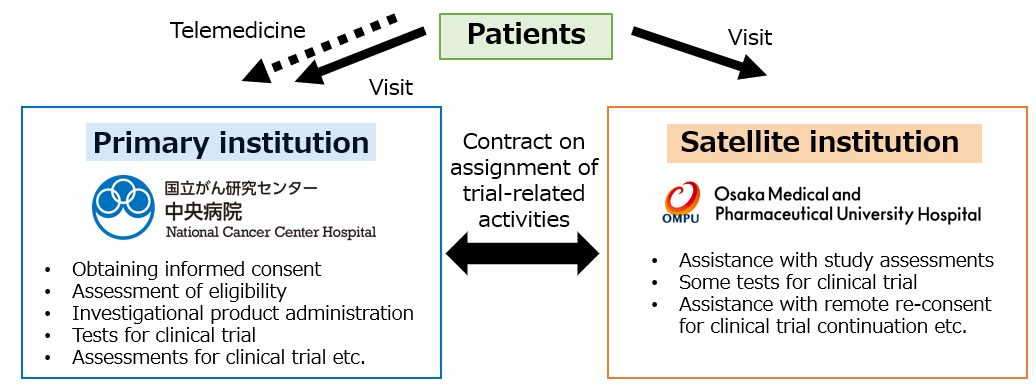
TOKYO, May 8, 2024 -- Chugai Pharmaceutical Co., Ltd. (TOKYO: 4519), National Cancer Center Hospital (hereafter, NCCH), Osaka Medical and Pharmaceutical University (hereafter, OMPU) and MICIN announced the introduce of a new decentralized clinical trial (DCT)[note1] structure and the start of DCT in a Chugai-sponsored phase I clinical trial for patients with advanced solid tumors. NCCH and OMPU will collaborate using telemedicine as the primary institution and the satellite institution, respectively, and some tests and assessments that were conventionally performed at institutions will be performed at the satellite institution [note2].
This is the first study in Japan to utilize a satellite institution for a company-sponsored phase I clinical trial in oncology. Since the number of institutions is very limited in phase I clinical trials, it is expected to improve access to clinical trials of new drug candidates for patients living far from institutions. We will evaluate this new DCT structure, with the aim of building an implementation structure that provides access to clinical trials for many patients regardless of where they live.
[note1] Decentralized clinical trial is a new approach to a clinical trial that is not dependent on visits to an institution. Utilizing digital technology to perform tests and assessments for a clinical trial being conducted at an institution gives benefits for patients by participating in a clinical trial without the need for regular in-person visits.
[note2] A satellite institution is a medical institution that is used when some tests and assessments for a clinical trial are performed outside of an institution.
Background and details
Since clinical trials of new drug candidates are conducted at a limited number of medical institutions, some patients living far from the institution give up their participation in the clinical trial due to the time and financial burden associated with in-person visits. Establishing an environment that improves the patients’ access to clinical trials is a common challenge in clinical development, including pharmaceutical companies and academias.[1] A retrospective study conducted at NCCH also showed that the participation rate in clinical trials tended to decrease for patients with travel time of 120 minutes or more.[2] In addition, in phase I clinical trials evaluating drug safety for cancer patients, it is necessary to closely monitor patients' condition while ensuring the patients' safety. Therefore, there are few medical institutions where phase I clinical trials can be conducted.
DCTs, which are not dependent on in-person visits, have been attracted attention as a new approach in recent years. In Japan, some guidelines have been issued, and DCT is gradually being introduced into clinical trials, but the use in the oncology area is still limited.
This is a Chugai-sponsored phase I clinical trial for patients with advanced solid tumors, conducted with NCCH as a primary institution and OMPU as a satellite institution. Since the travel time from OMPU to NCCH exceeds 120 minutes, DCT is expected to reduce patients’ burdens. Patients will be able to visit the satellite institution instead of the primary institution to receive some tests and assessments for this clinical trial via telemedicine. The DCT platform MiROHA, provided by MICIN, will also be utilized for telemedicine visits and for obtaining remote re-consent using eConsent.
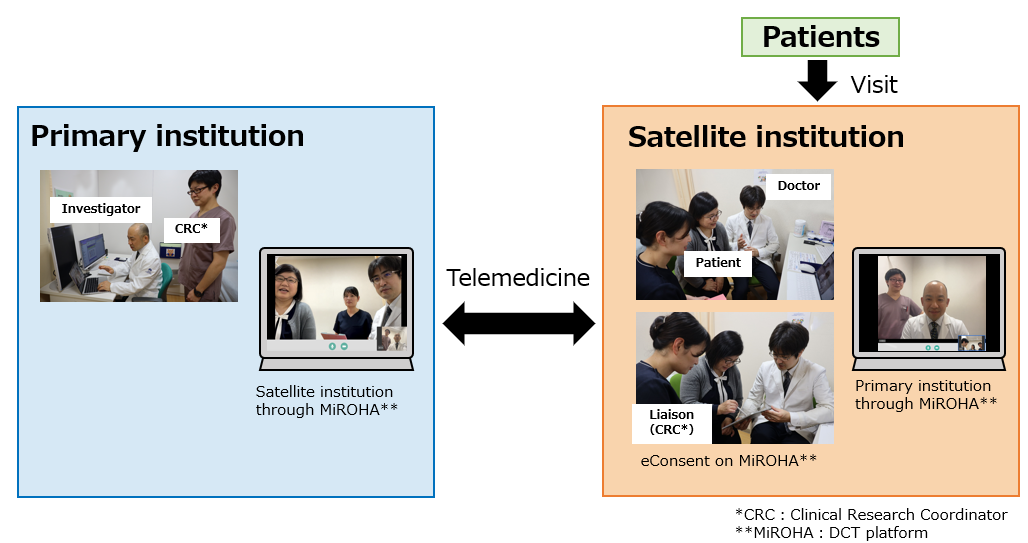
Comments
Dr. Osamu Okuda, President and CEO, Chugai Pharmaceutical Co., Ltd.
Improving access for patients is a common challenge in the pharmaceutical industry because clinical trials of new drug candidates are conducted at a limited number of medical institutions. The utilization of satellite institutions is expected to reduce the burden on patients and improve access to clinical trials. Chugai’s most prioritized value is patient-centricity. We will develop new drugs together with patients as partners, with the aim of realizing advanced and sustainable patient-centric healthcare.
Dr. Yasuyuki Seto, Director of National Cancer Center Hospital
To patients living in remote areas, access to clinical trials is drastically reduced, especially true for rare cancers and those of rare fractions, which trials are concentrated in hospitals in urban centers. By removing the need for patients to commute long hours, DCTs can address this issue. With new means, our team will reinvigorate drug development in Japan, delivering treatments expeditiously to patients nationwide, which is our mission.
Dr. Takahiro Katsumata, Osaka Medical and Pharmaceutical University Hospital
In the phase I clinical trial for solid tumors, our institution will play a role as a satellite institution, contributing to the implementation of "no one left behind in cancer care" practices. In the face of disparities in access to clinical trials and information, particularly in rural areas, we have established a decentralized clinical trial system aimed at reducing the burden on patients while addressing these disparities. We have experience in many phase I trials of new anti-cancer drugs, and we are committed to the success of this challenging trial and will endeavor to ensure the realization of cancer patients' "Well Being" even in rural regions.
Dr. Seigo Hara, MICIN, Inc., Representative CEO
It is hoped that this trial initiative, which utilizes satellite medical institutions, will reduce the burden on patients and expand their options for participating in clinical trials.
As a leading DCT company, we are committed to contributing to a new way of conducting clinical trials through this new attempt.
Reference
[1].Consideration of utilization of clinical trial methods not dependent on visits to institutions - guidance for introduction in Japan/Task Force 3 of Clinical Evaluation Subcommittee of Drug Evaluation Committee, Japan Pharmaceutical Manufacturers Association (July 2021) (Japanese only)
[2].Y Uehara, T Koyama, Y Katsuya, et al. Travel Time and Distance and Participation in Precision Oncology Trials at the National Cancer Center Hospital. JAMA Netw Open. 2023;6(9): e2333188
Company/institution information
About Chugai
Chugai Pharmaceutical Co., Ltd., headquartered in Tokyo, is a research-based pharmaceutical company with world-class drug discovery capabilities, including proprietary antibody engineering technologies. Chugai is committed to creating innovative pharmaceutical products that may satisfy unmet medical needs. Chugai is listed on the Prime Market of the Tokyo Stock Exchange. While maintaining autonomy and management independence, Chugai is an important member of the Roche Group. Additional information is available at https://www.chugai-pharm.co.jp/english/.(linked at external site)
About National Cancer Center Hospital
National Cancer Center Hospital upholds the vision ‘working with society, providing best suited cancer medicine to all citizens,’ thus leading the nation in cancer medicine, research and education. It is where the largest number of industry sponsored, investigator sponsored trials in Japan are ongoing, leading the cancer research community.
More information https://www.ncc.go.jp/en/ncch/index.html
About Osaka Medical and Pharmaceutical University Hospital
Osaka Medical and Pharmaceutical University Hospital is both a specialized medical facility providing advanced healthcare and the last stronghold of regional medicine in the north area of Osaka prefecture. The hospital's main building, which houses the newly established Cancer Center with state-of-the-art equipment introduced in July 2022, has been completed. Construction of the main building B is underway, aiming for its opening in July 2025. With its comprehensive facilities and functions, the hospital aims to provide patients and their families with medical care and comfort that cannot be found elsewhere.
About MICIN
MICIN is a medical startup with a vision of “For All To Live Out Their Lives With Dignity”.We provide telemedicine services to medical institutions and pharmacies, develop DTx(Digital Therapeutics), digital solution services for DCT(Decentralized Clinical Trial)and insurance services.
https://micin.jp(linked at external site)
MiROHA - A DCT Platform
The MiROHA system, a DCT platform provided by MICIN, is a system equipped with telemedicine, eConsent, and eSource (Electronic Source Data: electronic data that can serve as source documents for clinical trials) functions that has been available since April 2020.Currently, MiROHA has already been used by over 2,000 patients at more than 180 medical institutions in Japan.As a leading company in the DCT field, we not only provide DCT solutions, but also actively support and consult companies that are newly involved in DCT.
https://www.miroha.co/(linked at external site)
Contact information
Chugai Pharmaceutical Co., Ltd.
Contact from the press
Media Relations Group, Corporate Communications Dept.
Tel: +81-3-3273-0881
E-mail: pr●chugai-pharm.co.jp
Contact from investors
Investor Relations Group, Corporate Communications Dept.
Tel: +81-3-3273-0554
E-mail: ir●chugai-pharm.co.jp
National Cancer Center
For patients
National Cancer Center Hospital Department of Experimental Therapeutics Takafumi Koyama
Tel: +81-3-3542-2511 (Representative)
E-mail: takoyama●ncc.go.jp
Contact from the press
Office of Public Relations, Strategic Planning Bureau
Tel: +81-3-3542-2511 (Representative)
E-mail: ncc-admin●ncc.go.jp
Osaka Medical and Pharmaceutical University
Planning and Public Relations Section
Department of General Affairs
E-mail: hojin-koho●ompu.ac.jp
MICIN, Inc.
Sayaka Shinohara
Public Relations
E-mail: pr●micin.jp
