Home > Information > press release > Asian multicenter prospective study (CHOICE study) to elucidate the pathogenesis of cholangiocarcinoma(including FGFR2-rearranged) launched
Asian multicenter prospective study (CHOICE study) to elucidate the pathogenesis of cholangiocarcinoma(including FGFR2-rearranged) launchedA project promoting rare cancer research and development in the Asia-PacificThe first large-scale study in MASTER KEY Asia
December 8th, 2022
National Cancer Center Japan
Highlights
- A global study aiming at elucidating the pathogenesis of fibroblast growth factor receptor (FGFR) 2-rearranged (Note 1) cholangiocarcinoma is launched in Asian countries.
- Biliary tract cancer is classified as a rare cancer and with a relatively high incidence in the Asia-Pacific. Data on patients with biliary tract cancer will be collected not only from Japan but also from many other Asian countries to elucidate its pathogenesis.
Summary
A global study led by the Hospital (NCCH; director: Kazuaki SHIMADA) of the National Cancer Center (president: Hitoshi NAKAGAMA, Chuo-ku, Tokyo) is launched in Asian countries, including Malaysia, Thailand, the Philippines, Viet Nam, and Taiwan, towards elucidating the pathogenesis of FGFR2-rearranged cholangiocarcinoma.
In this study, tissue samples are collected from patients and checked comprehensively for genetic alterations using gene profiling approaches. Fluorescence in situ hybridization (FISH) (Note 2) is also used to detect the expression of FGFR2 fusion genes. Furthermore, the study will analyze the genetic information, together with clinical information such as treatment and prognosis to help figure out the pathogenesis, contributing to the development of therapeutics. We especially expect that the study will expand use of FGFR inhibitors in Asia.
Background
Biliary tract cancer affects a relatively large number of the Asian population and is classified as a cancer with poor prognosis because it is difficult to treat. However, several new target molecules have been recently discovered, giving us a clue for improving therapeutic approaches for this cancer. The development of therapeutics targeting the FGFR2 fusion gene, one of the target molecules, is ongoing mainly in Europe, the US, and Japan. Although frequently reported in the Asia-Pacific, data on biliary tract cancer are insufficient in this area, and more studies are needed to elucidate the genetic background of the majority of patients with biliary tract cancer.

This study is conducted as an Asian multicenter prospective study to comprehensively investigate FGFR2 fusion genes and other genetic alterations in patients with metastatic and/or recurrent cholangiocarcinoma, “Molecular detection and clinicopathological characteristics of advanced/recurrent CHOlangiocarcinoma harboring FGFR2 rearrangements In Asian CountriEs: CHOICE study,” as part of the NCCH initiative for promoting rare cancer research and development and genomic medicine in Asia, “Marker Assisted Selective ThErapy in Rare cancers: Knowledge database Establishing registry, Asia; MASTER KEY Asia” (Note 3).MASTER KEY Asia, a global study in Asia, is built onto the “MASTER KEY Project” (Note 4) launched in 2017 in Japan for driving research and development on rare cancers and for promoting genomic medicine, and is a successful collaboration between industry and academia. MASTER KEY Asia has two major purposes: (1) to comprehensively collect genetic information on patients with rare cancers and their treatment and prognosis and establish a large-scale database serving as basic study data and (2) to conduct investigator- or industry- initiated trials in patients with specific biomarkers (including genetic abnormalities and protein expression), irrespective of cancer type.
The CHOICE study will collect data on patients with cholangiocarcinoma classified as a rare cancer not only in Japan but also in many other Asian countries, contributing to the elucidation of its pathogenesis. We believe that the study will give hope for improving treatment of patients suffering from this cancer in the Asian region, including Japan.
Study procedures
In this study, tumor tissue samples will be collected from 150 patients with intrahepatic and hilar cholangiocarcinoma classified as biliary tract cancer often affecting Asian people, and then, DNA and RNA extracted from these tumor tissue samples will be comprehensively analyzed. Sites involved in the MASTER KEY Asia project will participate in the study, and cancer gene panel testing (TOP2 Panel) (Note 5) enabling the detection of at least 700 cancer-associated genetic alterations will be used for genetic analysis. FISH using tumor tissue will also be used to detect specific genes called FGFR2 fusion genes to evaluate concordance with analysis results of cancer gene panel testing. Furthermore, it is known that liver fluke (Note 6) infection is associated with the onset of biliary tract cancer in the Asian population; hence, a history of parasite infection will also be investigated.
Genetic analysis results and the clinical information of the patient will be pooled for analysis to determine the frequency of FGFR2 fusion genes and encourage the development of therapeutics using molecular target drugs.
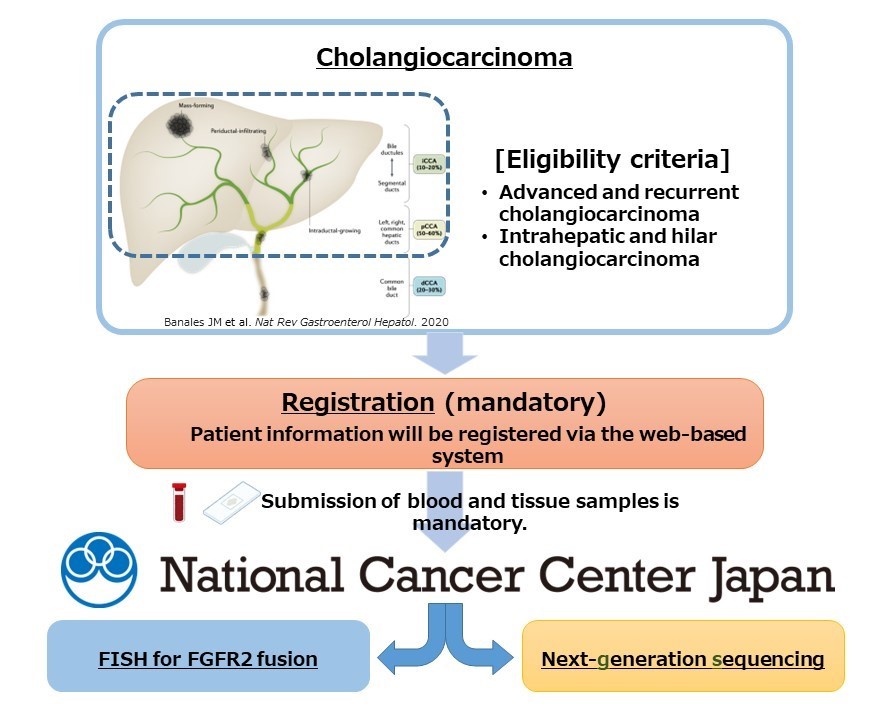
Figure: Study Methods
Results obtained from this study will be fed back to physicians in each site. Although we will not conduct a study to administer FGFR inhibitors to patients with FGFR2 fusion genes, this study may help recommend medication use depending on the approval status in each country and may be useful in treating patients.
Effects of this study
This study involves not only the identification of FGFR2 fusion genes but also the implementation of genetic testing using TOP2 Panel, which enables us to evaluate various genetic alterations profiles in biliary tract cancer (intrahepatic and hilar cholangiocarcinoma). The study findings may lead to the accumulation of useful data for developing novel drugs and expanding the market for existing molecular target drugs. We hope that the study will provide more treatment options for Japanese and Asian patients suffering from this disease.
Research project members
National Cancer Center Hospital
Takuji Okusaka (principal investigator), Yuta Maruki, Department of Hepatobiliary and Pancreatic Oncology
Hitomi Okuma, Department of Medical Oncology
Yasushi Yatabe, Department of Diagnostic Pathology
Kenichi Nakamura, Chiharu Mizoguchi, Department of International Clinical Development
Tetsuya Sasaki, International Trial Management Section, Research Management Division, Clinical Research Support Office
Funding
The study is partially funded by the Japan Agency for Medical Research and Development (AMED) for the project for promoting clinical research and trial activities, “Asian Clinical Trials Network for Cancers Project” (representative: Kazuaki SHIMADA, director of NCCH) (Note 7). Eisai Co., Ltd. also funded the study.
Glossary
(Note 1) FGFR2 fusion genes
FGFR, fibroblast growth factor receptor, is a protein in the cell membrane.FGFR gene aberration includes fusion, mutation, and amplification. Once function of FGFR with gene aberration is activated, it may lead to cancer cell growth, survival, and migration; tumor angiogenesis; drug resistance; and other problems.FGFR gene aberration has been reported for various tumors, including lung, breast, endometrial, gastric, and bladder cancers; cholangiocarcinoma; and brain tumor.
(Note 2) FISH (fluorescence in situ hybridization)
FISH is a technique that uses a fluorescent probe (a synthetic gene with a base sequence complementary to the target gene) bound to the target gene to visualize genetic materials under a fluorescence microscope.
(Note 3) MASTER KEY Asia

MASTER KEY Asia is a global, prospective registry study on rare cancers with small patient populations, for which the development of therapeutics is difficult to achieve, in 10 sites in 5 Asian countries (Malaysia, Thailand, Indonesia, the Philippines, and Viet Nam).
For more information, please see the following press release:
The press release from the National Cancer Center Japan on October 20, 2021
A New Asia-Pacific Collaboration MASTER KEY ASIA Promoting genomic medicine throughout Asia
(Note 4) MASTER KEY Project
Rare cancers are cancers with small patient populations, which makes it difficult to conduct research and clinical studies because of the limited amount of clinical data from individual patients. The “MASTER KEY Project” is the first attempt in the world to solve the issue associated with rare cancers in collaboration with NCC and pharmaceutical companies to promote genomic medicine for rare cancers.
The MASTER KEY PROJECT
For more information, please see the following press release:The press release from the National Cancer Center Japan on July 31, 2017
The MASTER KEY PROJECT Genomic medicine in Rare Cancers:A collaboration between the industry and academia
(Note 5) TOP2 Panel
TOP2 Panel is a new cancer gene panel test that enables the detection of at least 700 genetic abnormalities.
(Note 6) Liver fluke
Liver fluke is a parasite associated with freshwater fish found mainly in Asia. It also infects humans who eat undercooked freshwater fish. An association between Opisthorchis viverrini and cholangiocarcinoma has been epidemiologically demonstrated.
(Note 7) Asian Clinical Trials Network for Cancers Project (ATLAS Project)
ATLAS Project started in September 2020 with the support of the Japanese government and AMED. This project aims to upgrade the soft infrastructure, such as education and training and improve the hard infrastructure, in terms of clinical trials. In addition, Asian Partnerships Office of NCC was established to conduct several global collaborative research projects and to achieve face-to-face communication with liaison officers at each region. Utilizing the framework of ATLAS Project, NCC plans to conduct a minimum of five global studies in the Asian region at all times, inviting global stakeholders, such as pharmaceutical companies, CROs, and patient advocates, to join this scheme.
Contact
Study Inquiries
Tetsuya Sasaki
International Trial Management Section, Research Management Division, Clinical Research Support Office, National Cancer Center Hospital
Yuta Maruki (ymaruki@ncc.go.jp)
Department of Hepatobiliary and Pancreatic Oncology
Tel: +81-3-3542-2511 (main); E-mail: ncch2007_office●ml.res.ncc.go.jp
Media Inquiries
Office of Public Relations, Strategic Planning Bureau, National Cancer Center Japan
Tel: +81-3-3542-2511 (main); E-mail: ncc-admin●ncc.go.jp


