Home > World's Largest-Scale Integrated Analysis Confirmed Prolonged Survival with Personalized Precision Oncology
World's Largest-Scale Integrated Analysis Confirmed Prolonged Survival with Personalized Precision Oncology- SCRUM-Japan MONSTAR Project Publishes Research Results in the AACR's Flagship Journal, "Cancer Discovery" -
In Japanese
Highlights
- An integrated analysis of molecular profiles, treatment outcomes, and survival data was conducted on 16,144 patients who participated in four multicenter studies under the SCRUM-Japan MONSTAR-SCREEN project (hereafter referred to as the MONSTAR project), the first industry-academia collaborative nationwide cancer genome screening project in Japan targeting a wide range of solid cancers.
- Patients in the MONSTAR Project who received targeted therapies matched to biomarkers identified through molecular profiling had significantly longer survival compared to those who did not receive matched therapies, confirming the significance of biomarker-based "personalized precision oncology".
- Currently, research on molecular profiling of cancer including RNA analysis (transcriptomics), protein analysis (proteomics), and metabolite analysis (metabolomics) as well as genome analysis, is progressing.
- MONSTAR project plans to launch a new large-scale study that will introduce multi-omics analysis using cutting-edge molecular profiling technologies to further develop personalized precision oncology.
Summary
A research group led by Dr. Takayuki Yoshino, Deputy Director and Dr. Tadayoshi Hashimoto, Gastrointestinal Oncology/Translational Research Support Office, National Cancer Center Hospital East (Director: Dr. Toshihiko Doi) at the National Cancer Center Japan (President: Dr. Hitoshi Nakagama, Chuo-ku, Tokyo), conducted an integrated analysis of 16,144 patients who participated in four studies (GI-SCREEN*1, GOZILA*2, MONSTAR-SCREEN*3, and MONSTAR-SCREEN-2*4) under the MONSTAR project*5. The analysis involved detailed examination of patients' cancer molecular profiles*6, the proportion of patients who received investigational drug treatments based on biomarkers, treatment outcomes, and survival of patients who received biomarker-based targeted therapies.
The results showed that among patients (n=674) who received investigational drug treatments, 29.2% (n=197) achieved tumor shrinkage of 30% or more (response), with a median overall survival of 14.8 months, indicating extended outcomes. Furthermore, approximately 20% of all patients in this study received targeted therapies matched to their biomarkers, and their survival was longer compared to those who did not receive matched therapies.
These findings underscore the importance of biomarker-based cancer precision medicine. It is expected that the MONSTAR project will further advance cancer precision medicine by leveraging multi-omics analysis*7 technologies, ultimately delivering effective treatments to more cancer patients in a timely manner.
The study results were published in the American Association for Cancer Research's flagship journal, "Cancer Discovery", on July 18, 2024 (Japan time).
Background
In recent years, molecular profiling technologies that examine the detailed genetic and molecular characteristics of cancer have greatly advanced, leading to the development of personalized precision oncology. The National Cancer Center Hospital East launched SCRUM-Japan, an industry-academia collaborative nationwide cancer genome screening project, in 2015. In collaboration with medical institutions and pharmaceutical companies across Japan, efforts have been made to conduct molecular profiling of cancer patients. The purpose of this project is to deliver more effective treatments to as many cancer patients as quickly as possible. The MONSTAR Project has conducted various large-scale studies targeting solid cancer patients other than those with lung cancer. To date, more than 24,000 patients have participated in these studies, and a database has been created that combines the results of multi-omics analyses examining genetic and molecular characteristics with detailed clinical information. There have been few reports evaluating the effectiveness of investigational drugs and the efficacy of biomarker-based therapies using data from such large-scale projects. In this study, the results of four studies conducted under the MONSTAR Project were integrated, and one of the world's largest-scale analyses was performed.
Research methods and results
In this study, an integrated analysis was conducted on data from 16,144 patients who participated in four large-scale clinical studies under the MONSTAR Project: GI-SCREEN, GOZILA, MONSTAR-SCREEN, and MONSTAR-SCREEN-2. The analysis examined the characteristics of patients who participated in clinical trials, the types of biomarkers targeted for treatment, and the effectiveness of therapeutic agents. The results showed that 5.0% of all patients participated in clinical trials, with a higher proportion of patients with colorectal cancer (51.8%), biliary tract cancer (9.5%), gastric cancer (9.0%), esophageal cancer (5.3%), and pancreatic cancer (5.0%). The most common timing for participating in clinical trials was fourth-line treatment or later (46.4%), while participation in first-line treatment was low (9.6%). Among patients (n=674) who received investigational drug treatments, 29.2% (n=197) had tumor shrinkage of 30% or more (response), and the median overall survival was 14.8 months, indicating extended outcomes. The most effective investigational drugs were anti-HER2 therapies*8 (Figure 1).
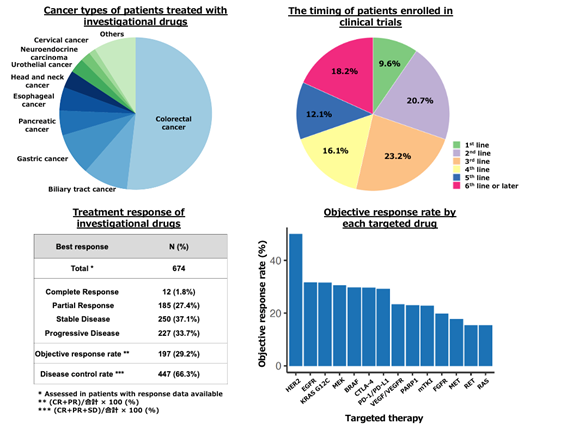
Figure 1: Treatment Analysis of Patients Who Participated in Clinical Trials from the MONSTAR Project
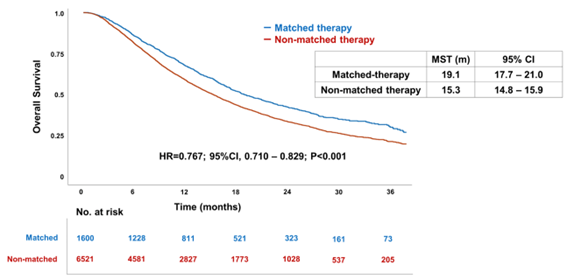
Figure 2: Survival Analysis of Patients Receiving Matched and Unmatched Therapies Based on Biomarkers
These results indicate that based on the molecular profiling conducted in the MONSTAR Project, many patients received investigational drug treatments with effective outcomes. Moreover, it was suggested that biomarker-matched targeted therapies may improve patient prognosis.
Future Perspectives
The findings of this study clearly demonstrate the importance of personalized precision oncology that delivers targeted therapies matched to biomarkers identified through various molecular profiling tests. Recent remarkable advancements in analytical and drug discovery technologies have enabled precise molecular profiling that examines the characteristics of each patient's tumor in greater detail. By leveraging these cutting-edge technologies, it is highly anticipated that more effective treatments can be delivered to more cancer patients, faster.
The MONSTAR Project plans to launch a new large-scale study called "MONSTAR-SCREEN-3." This study will expand its scope to include not only patients with advanced solid tumors but also those with early-stage solid tumors amenable to curative resection and patients with hematologic malignancies. The study will conduct state-of-the-art multi-omics analyses tailored to each disease condition. We will continue to utilize the world's most advanced multi-omics analyses and dedicate our efforts to the development of personalized precision oncology, aiming to deliver effective treatments to cancer patients and their families worldwide.
For MONSTAR Project execution, we would like to express our deepest gratitude to all the patients and their families who cooperated with our research, to all the medical institutions nationwide, and to the many stakeholders involved in the operation of the project.
About MONSTAR Project
The MONSTAR Project is the first industry-academia collaborative nationwide cancer genome screening project for personalized precision oncology in Japan that aims to deliver effective diagnostic and therapeutic agents to participating patients as quickly as possible, and numerous studies have been conducted to date. This project is supported and operated by multiple committees consisting of researchers and medical professionals from various specialized fields. These studies collect the genomic and molecular characteristics of cancer as well as the detailed clinical information about the treatment and its effectiveness over the long term, and the data is securely stored in a supercomputer called "KASHIWARP" installed at the National Cancer Center Hospital East. This supercomputer is equipped with various cutting-edge software, including artificial intelligence-based analyses, enabling high-speed and efficient analysis of vast amounts of data (Figure 3).
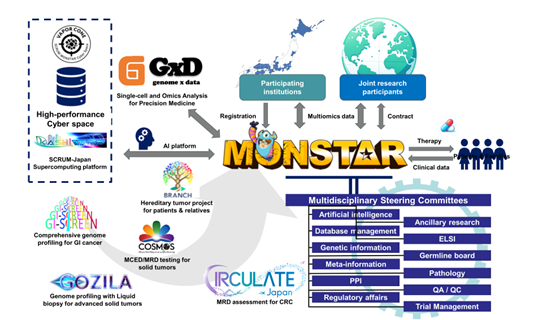
Figure 3: Overview of the SCRUM-Japan MONSTAR Project
Publication
Journal
Cancer Discovery
Title
The SCRUM-MONSTAR Cancer-Omics Ecosystem: Striving for a Quantum Leap in Precision Medicine
Authors
Tadayoshi Hashimoto, Yoshiaki Nakamura, Takao Fujisawa, Mitsuho Imai, Taro Shibuki, Naoko Iida, Hiroshi Ozaki, Norio Nonomura, Chigusa Morizane, Hiroji Iwata, Susumu Okano, Wataru Yamagami, Naoya Yamazaki, Shigenori Kadowaki, Hiroya Taniguchi, Makoto Ueno, Shogen Boku, Eiji Oki, Yoshito Komatsu, Satoshi Yuki, Akitaka Makiyama, Tomoyuki Otsuka, Hiroki Hara, Naohiro Okano, Tomohiro Nishina, Yasutoshi Sakamoto, Izumi Miki, Shin Kobayashi, Junichiro Yuda, Shun-Ichiro Kageyama, Michiko Nagamine, Shingo Sakashita, Naoya Sakamoto, Riu Yamashita, Yoshikatsu Koga, Hideaki Bando, Genichiro Ishii, Takeshi Kuwata, Woong-Yang Park, Atsushi Ohtsu, Takayuki Yoshino*
*Corresponding author
DOI
10.1158/2159-8290.CD-24-0206
Date
July 18, 2024 (Japan time)
URL
https://aacrjournals.org/cancerdiscovery/article/doi/10.1158/2159-8290.CD-24-0206/746433/The-SCRUM-MONSTAR-Cancer-Omics-Ecosystem-Striving (linked at external site)
Glossary
*1 GI-SCREEN (2015-2019)
A multicenter collaborative cancer gene screening study targeting gastrointestinal cancer patients. It is a nationwide cancer genome screening project that analyzes tumor tissues of advanced gastrointestinal cancer patients using gene panel testing (Oncomine Comprehensive Assay) and delivers therapeutic agents. A total of 5,743 patients was enrolled in the project.
*2 GOZILA (2018-2024)
A project that conducts screening using blood-based genetic analysis (liquid biopsy) for patients with advanced gastrointestinal cancers. The highly sensitive genetic analysis technology Guardant360® assay developed by Guardant Health, Inc. in the United States was used. Currently, approximately 5,500 patients have been enrolled in the project. (Registration has been completed, with some related studies remaining.)
*3 MONSTAR-SCREEN (2019-2021)
A screening project that conducts testing and analysis of cancer tissues, liquid biopsies, and gut microbiome using fecal samples in a wide range of solid cancer patients. FoundationOne CDx technology was used for tissue analysis, and FoundationOne Liquid CDx was used for liquid biopsy. MONSTAR-SCREEN has expanded the target patient population from gastrointestinal cancers to all solid cancers other than lung cancer and conducts molecular profiling of cancers. A total of 2,224 patients was enrolled in the project.
*4 MONSTAR-SCREEN-2 (2021-2024)
A screening project that comprehensively analyzes abnormalities in cancer DNA, RNA, proteins, etc., using cancer tissues and liquid biopsies in patients with advanced solid tumors other than lung cancer. Given the vast amount of data obtained from these tests, artificial intelligence-driven analysis has also been introduced as a method to quickly process large quantities of information. A total of 2,768 patients was enrolled in the project.
*5 SCRUM-Japan MONSTAR-SCREEN
SCRUM-Japan is an industry-academia collaborative cancer genome screening project that integrates LC-SCRUM-Japan (currently LC-SCRUM-Asia), which started in 2013 targeting lung cancer patients, and GI-SCREEN-Japan (currently MONSTAR-SCREEN), which started in 2014 targeting gastrointestinal cancer patients.
*6 Molecular Profile
A molecular profile refers to the detailed characteristics of cancer, which are revealed by closely examining changes in gene and protein expression. Examining this molecular profile in detail is called molecular profiling, and it provides important information for selecting personalized treatment for each patient by understanding the characteristics of each patient's cancer.
*7 Multi-omics Analysis
A method that comprehensively analyzes genetic analysis (genomics), RNA analysis (transcriptomics), protein analysis (proteomics), metabolite analysis (metabolomics), and more. The term "-omics" signifies a comprehensive analysis.
*8 Anti-HER2 Therapy
HER2 (human epithelial growth factor receptor type 2) is a protein located on the cell membrane that belongs to the HER family. HER2 protein is involved in cell proliferation in normal cells, but when HER2 protein is overexpressed, or HER2 gene amplification occurs for some reason, it is thought to lead to cancer as cell proliferation can no longer be controlled. HER2 protein overexpression has been confirmed in multiple cancers, and drugs targeting HER2 protein have already been approved in Japan. HER2 is considered a promising molecular target for cancer treatment.
Enquiries
On Research
SCRUM-Japan Office, National Cancer Center Hospital East
E-mail: scrum_office●east.ncc.go.jp
From the Media
Office of Public Relations, Strategic Planning Bureau,
National Cancer Center (Kashiwa Campus)
E-mail: ncc-admin●ncc.go.jp
