Home > Specialist ctrs & depts. > Clinical Research Support Office
Clinical Research Support Office
Noboru Yamamoto, Kenichi Nakamura
- Clinical Research Coordinating Division
Hideki Ueno, Miki Ito, Chie Miyano, Asako Sakamoto, Sho Murata, Mari Takahashi, Hiroko Kawaguchi, Shinobu Araki, Chiharu Nakano, Asako Kamikawa, Kimiyo Yoshii, Hiroko Takagi, Maki Takayasu, Aya Matsumoto, Yukako Takasaki, Ran Obara, Harue Ui, Kayo Mukai, Nahoko Hiraoka, Hatsuki Ohno, Chikano Yashiro, Yukari Nishiyama, Azumi Seko, Rie Goto, Marie Komagata, Aya Shiroichi, Anna Yashima, Miki Tamaki, Saki Yoshizawa, Yumi Nagashima, Yumi Tada, Asami Kaido, Chiaki Nishikawa, Marina Hirooka, Yuko Shinoda, Aya Kato, Natsumi Fujii, Maki Moriya, Sumika Oka, Kazumi Kojima, Kana Miura, Marie Ito, Ai Kobayakawa, Maya Niigawa, Hiroe Takayama, Mari Kondo, Tomoko Jinbo, Shiho Nishino, Chie Moteki, Kimiko Sega, Nobuko Ushirozawa, Yoshiko Kanazu, Yoko Ebihara, Harumi Maruno, Junko Horie, Eiko Mimata, Kaori Matsushita, Yuki Sezaki, Chiaki Yamamoto, Yoshiko Miyahara, Ken Kato, Keiko Wakakuwa, Mayumi Ikeda, Satomi Nakamori, Harumi Mochizuki, Azusa Komada
- Research Management Division
Natsuko Okita, Hisahiro Ito, Satoshi Kawashima, Masahiko Ichimura, Reiko Isomura, Namiko Kondo, Tetsuya Sasaki, Yayoi Ando, Takako Hitomi, Mamiko Kawasaki, Naoko So, Masako Inaba, Nami Shirakawa, Eri Tsutsui, Tomomi Hata, Hitomi Okuma, Chiharu Mizoguchi, Aya Miyata, Tomozo Yamada, Sachie Kawabata, Eiko Yorikane, Yoshie Shuda, Kenta Anjo, Kaori Izumino, Kota Yamagishi, Mamiko Nakata, Kazumi Ono, Anri Uchimura, Hiroshi Katayama, Keita Sasaki, Yusuke Sano, Noriko Mitome, Kota Kawabata, Ayaha Kuriyama, Emi Iwata, Takesi Uozumi, Hisako Oi, Yuko Tominaga, Naoko Murata, Ryosuke Kita, Tetsuya Sekita, Junki Mizusawa, Gakuto Ogawa, Ryunosuke Machida, Masayuki Yokoyama, Yuya Ishikawa, Taro Shibata, Aya Kuchiba, Shogo Nomura, Akihiro Hirakawa, Ryo Sadachi, Kohei Uemura, Riku Kajikawa, Naomi Konishi, Yuko Minami, Yuki Konda
- Data Management Division
Haruhiko Fukuda, Harumi Kaba, Hiromi Katsuki, Yumi Oshikiri, Yayoi Mitsumori, Tomoko Kojima, Junki Mizusawa, Nobuko Okamura, Ryuji Makiuchi, Keiko Ohata, Yukari Hoshina, Masahisa Kamikura, Yukari Nagasaka, Kazumi Kurishita, Mai Naito, Takashi Fuwa, Tomoko Takayama, Ayako Kumazawa, Emi Nakamura, Keiko Suto
Introduction
The Clinical Research Support Office supports clinical research conducted under the leadership of investigators in the National Cancer Center Hospital (NCCH). Support activities include protocol writing, central/local data management, statistical design and analysis, in-house/on-site monitoring, audits, patient recruitment, and other coordinating jobs. We also provide local support for industry-sponsored registration-directed trials and investigator-initiated registration-directed trials conducted at the NCCH.
The Team and What We Do
- Clinical Research Coordinating Division
The Clinical Research Coordinating Section and the Clinical Trial Administration Section support a lot of industry-sponsored registration-directed trials as well as investigator-initiated registration-directed trials.
The Biobank and Translational Research Support Section has routinely obtained informed consent to participate as an NCC biobank (NCCBB) donor from patients who consult with the NCCH for the first time. CRCs in this section coordinate translational research in several ways, such as assistance for registration for clinical trials, logistics for pathological specimens, data collection for case report forms, and coordination between sections.
- Research Management Division
The Research Management Division is in charge of central research support functions: i)Promotion of digital transformation (DX) , ii) Investigator-initiated registration-directed trial (Chiken) management, iii) Monitoring & Investigator-initiated registration-directed trial(Advanced Medical Care), iv) Multi-institutional clinical trial support, v) Biostatistics, vi) Promotion of Medical Device Development & Pharmaceutical affairs consultation. The division has the capability to support various types of clinical trials covering both early phase ones including first-in-human trials and late phase multi-institutional trials. For the early phase trials, the division mainly offers comprehensive study management, site visit monitoring and safety information management. One of the strengths of the division is that it can coordinate not only domestic trials but also international investigator-initiated registration-directed trials. The multi-institutional trial support function works as the Japan Clinical Oncology Group (JCOG) Operations Office which engages in protocol development, manuscript drafting, study coordination, etc. for late phase trials.
- Data Management Division
The Data Management Division is responsible for central data management and in-house study monitoring in investigator-initiated clinical trials for cancer therapeutic development. The Data Management Section supports early phase cancer trials mainly for drug development including registration trials which are led by physicians in the NCCH. The Multi-institutional Data Management Section supports mostly late development multi-modality multi-institutional phase II or phase III trials for adult solid cancer conducted by the Japan Clinical Oncology Group (JCOG).
Clinical trials
- Clinical Research Coordinating Division
The number of registration-directed clinical trials is increasing year by year, and the division supported 511 registration-directed trials including 56 investigator-initiated registration-directed trials in FY 2023 (Table 1).
- Research Management Division
The priority area of the division is genomic medicine, rare cancer and international trials. As of the end of the fiscal year 2024, the Research Management Division supported 24 investigator-initiated registration-directed trials, 11 clinical trials using the Advanced Medical Care system and two trials under patient-proposed health services. This division has been in charge of the coordinating office of rare cancer registry study with basket sub-studies (MASTER KEY Project). This division also supports an international investigator-initiated registration-directed trial (PATHWAY trial).
- Data Management Division
The Data Management Section supports 17 IND trials (7 open, 3 in preparation, 7 on follow-up) and 9 non-IND studies (5 open, 4 on follow-up). The Multi-institutional Data Management Section supports 2 open IND trials and 103 non-IND trials (47 open, 11 in preparation, 45 on follow-up) as the JCOG Data Center.
Table 1. Supported Trials in the Clinical Research Coordinating Division in FY 2023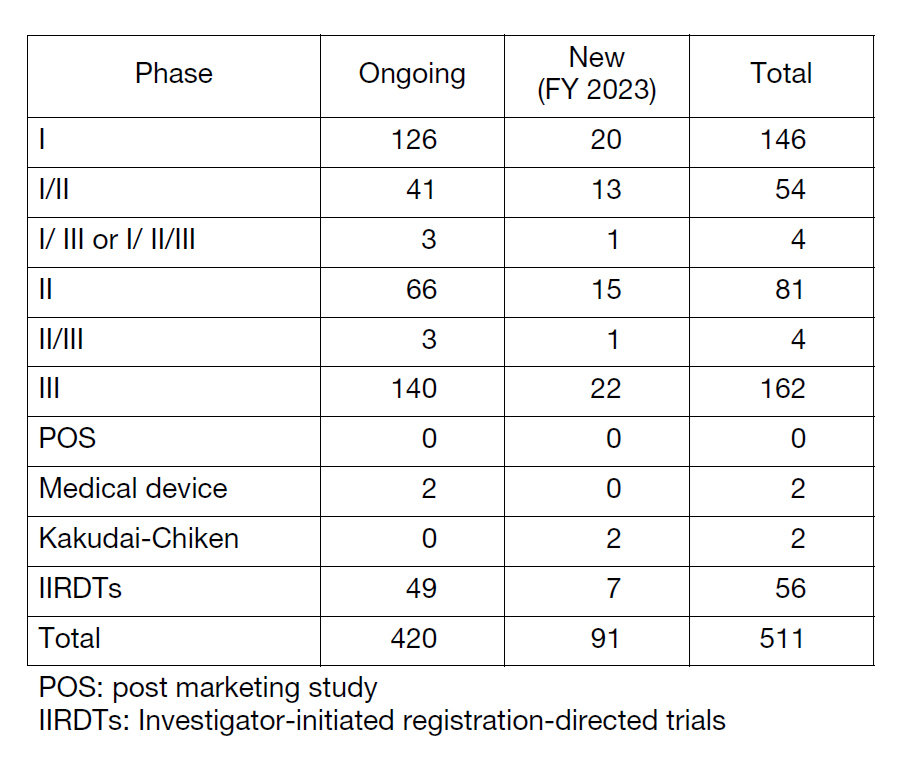
Education
- Clinical Research Coordinating Division
The staff members received not only day-to-day on-the-job training but also in-house educational seminars in order to learn how to support clinical trials including investigator-initiated registration-directed trials.
- Research Management Division
The staff members received not only day-to-day on-the-job training but also various seminars related to clinical trials held within the National Cancer Center or scientific meetings in order to learn how to support clinical trials including investigator-initiated registration trials.
- Data Management Division
Data managers in the division are encouraged to attend the various seminars related to research ethics and clinical trials held within the National Cancer Center, in-house lectures organized by the Clinical Research Support Office, scientific meetings and seminars organized by the Japan Society of Clinical Trials and Research or the Ministry of Health, Labour and Welfare and so on. The division is also continuously promoting on-the-job training of data managers to teach them clinical trial methodology, data management and research ethics.
Future Prospects
- Clinical Research Coordinating Division
The number of supported clinical trials is increasing as previously described, and the supported area covered by CRCs will be expanded to include not only registration trials but also other investigator-initiated clinical trials. Therefore, expansion of CRC staff members is highly anticipated. In view of the plan for the NCCH, all members of this office will work together to contribute to reinforcing clinical research capabilities of the NCCH and to making this office a valuable unit for all members of our hospital.
The members of the Biobank and Translational Research Support Section received seminars which are related to clinical research ethics, etc. The section informs and educates investigators of the NCC about the NCC biobank and translational research periodically through the NCC University. As a future direction, the section will improve the quality of the NCC biobank’s informatics and storage of serum. The section also aims to establish a support system for higher quality and quantity.
- Research Management Division
Since the number of investigator-initiated registration-directed trials has increased, reinforcement of staff resources is urgently needed. In response to this increase, this division will reinforce the support function for various clinical trials including international clinical trials, investigator-initiated registration-directed trials, Advanced Medical Care system trials, patient-proposed health services trials, and a new type of clinical trial (platform trials). Also, this division will establish optimal ways to cope with the Clinical Research Act and revised Ethical Guidelines.
- Data Management Division
The Data Management Division has introduced a web-based electronic data capturing (EDC) system and is promoting standardization of all aspects of data management, such as data formats, case report forms and monitoring reports for increased data integrity, and cost effectiveness of day-to-day work.
