Home > Specialist ctrs & depts. > Screening Center
Screening Center
Staff
Nozomu Kobayashi, Masau Sekiguchi, Keiko Nakamura, Yasuo Kakugawa, Minori Matsumoto, Eriko Tsuruki, Masayoshi Yamada, Hiroyuki Takamaru, Takaaki Tsuchida, Masahiko Kusumoto, Gen Iinuma, Nachiko Uchiyama, Mari Kikuchi, Kimiteru Ito, Hiroaki Kurihara, Miyuki Sone, Yasunori Mizuguchi, Hirokazu Watanabe, Mototaka Miyake, Shunsuke Sugawara, Yuko Kubo, Chihiro Ito, Nao Kikkawa, Shintaro Kimura, Sawako Kaku, Mizuki Ozawa, Tomoyasu Kato, Mitsuya Ishikawa, Masaya Uno, Yasuhito Tanase, Mayumi Kato, Kenichi Nakamura, Hiroshi Katayama, Junko Eba, (Visiting Researcher) Takahisa Matsuda, Shuji Yamamoto, Hidetsugu Yamagishi, (Special Research Assistant) Mika Mori
Introduction
In the Cancer Screening Center (former name: Research Center for Cancer Prevention and Screening; RCCPS), we have provided broad opportunistic cancer screening using newly developed modalities since 2004. Most of the staff doctors hold two positions concurrently in both the Cancer Screening Center and their own specialized department. Our department is in charge of multiphasic cancer screening using several imaging modalities to develop new cancer screening systems and to assess new screening tests.
The Team and What We Do
- Cancer screening courses: The basic plan for males consists of screening for cancer of the lungs, esophagus, stomach, colorectum, liver, gall bladder, pancreas, kidneys, and prostate. The basic plan for females consists of screening for cancer of the breast, uterus, and ovaries, in addition to the plan for males, excluding the prostate. In addition, PET (positron emission tomography) is provided as an option. In addition to multiphasic programs, an independent cancer screening program has been prepared for cancers of the lungs and female genitalia, including cancer of the uterus, ovaries, breast, and gastrointestinal tract. Blood samples are also obtained for biochemistry and tumor markers such as CA19-9, CEA, CA125, and PSA, as well as for genetic analysis.
- Cancer screening methods: On the first day, the multiphasic cancer screening programs (comprehensive cancer screening program) perform CT for lung cancer, abdominal US for cancer of the liver, gall bladder, pancreas, and kidneys, gynecological examinations with pap-smear and HPV test for uterine cancer, and MMG/tomosynthesis and US for breast cancer. On the following day, gastroscopy for cancer of the esophagus and stomach, and total colonoscopy for cancer of the colon and rectum are conducted. Moreover, from the beginning of December 2010, CT-colonography (CTC) has been provided as an option for cancer screening. FDG-PET/CT is offered on the first day as an option, if the participants wish to undergo the examination. Furthermore, FDG-PET/MRI has been provided as an optional examination since 2018.
- Number of cancer screening participants: The number of cancer screening participants between April 2022 and March 2023 is shown in this report (Table 1). Due to the influence of COVID-19, the number of participants decreased remarkably. A total of 2,386 people underwent cancer screening at the Cancer Screening Center during this period. Most of the participants (90%; n=2,157) chose the comprehensive cancer screening course. Regarding the cancer detection rate data in each modality, we will report them in the near future.
Research activities
Amino-index (AICS) cancer screening accuracy evaluation study
Since the start of the research in July 2012, we have received plasma samples from 8,111 screening examinees and are proceeding with comprehensive amino acid analysis. This year, we confirmed the results of AICS in the patients with gastric cancer, using data of 6,532 individuals. Among thirty-three patients with gastric cancer, only five were classified as rank C, the highest risk group. The sensitivity and specificity of AICS for gastric cancer were 15% and 90%, respectively.
Study using colorectal cancer screening data from colonoscopy (CS)
The AP-Colon SL study is a prospective study of colorectal serrated lesions in patients undergoing colorectal cancer screening at multicenter sites in the Asia-Pacific region, aiming to determine the true prevalence of serrated lesions and to identify the risk factors for colorectal serrated lesions. For the total colonoscopy, the protocol stipulates that all patients will undergo right-sided colonoscopy with dye endoscopy using indigocarmine to more reliably detect serrated colorectal lesions and to determine their true prevalence. The trial is ongoing with a planned enrollment of 1,000 patients.
Clinical trials
We are conducting ongoing research based on the study protocol titled “Evaluation of effectiveness of cancer screening modality at the National Cancer Center”. The target modalities are as follows: 1) upper gastrointestinal endoscopy, 2) lower gastrointestinal endoscopy, 3) CT colonography, 4) chest computed tomography (CT), 5) sputum cytology, 6) mammography, 7) breast ultrasonography, 8) FDG-positron emission tomography (PET), 9) abdominal ultrasonography, and 10) serum tumor markers.
Future Prospects
Based on cancer screening data such as examination results, medical institution findings, follow-up findings, and the questionnaire survey concerning lifestyles for 10 years, we commenced with assessment them with the support of the National Cancer Center Research and Development Fund.
Figure
Table 1. Number of patients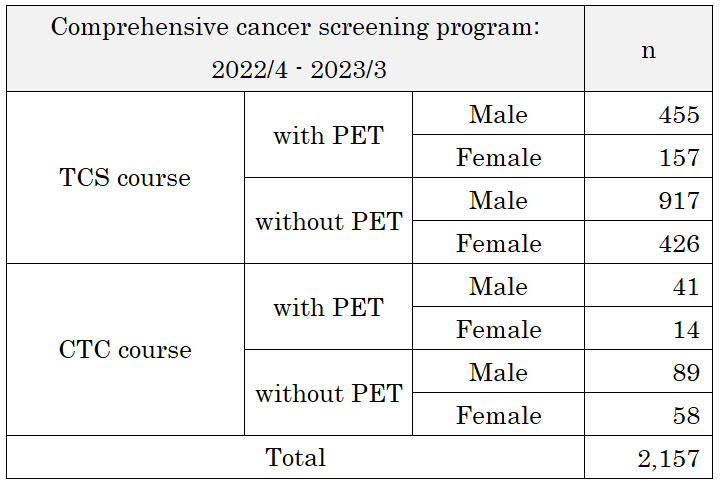
List of papers published
Journal
- Sano Y, Hotta K, Matsuda T, Murakami Y, Fujii T, Kudo SE, Oda Y, Ishikawa H, Saito Y, Kobayashi N, Sekiguchi M, Ikematsu H, Katagiri A, Konishi K, Takeuchi Y, Iishi H, Igarashi M, Kobayashi K, Sada M, Osera S, Shinohara T, Yamaguchi Y, Hasuda K, Morishima T, Miyashiro I, Shimoda T, Taniguchi H, Fujimori T, Ajioka Y, Yoshida S; Japan Polyp Study Workgroup. Endoscopic Removal of Premalignant Lesions Reduces Long-Term Colorectal Cancer Risk: Results From the Japan Polyp Study. Clin Gastroenterol Hepatol. 2024 Mar;22(3):542-551.
- Sekiguchi M, Igarashi A, Toyoshima N, Takamaru H, Yamada M, Esaki M, Kobayashi N, Saito Y. Cost-effectiveness analysis of computer-aided detection systems for colonoscopy in Japan. Dig Endosc. 2023 Nov;35(7):891-899.
- Sekiguchi M, Matsuda T, Saito Y. Treatment strategy and post-treatment management of colorectal neuroendocrine tumor. DEN Open. 2023 Jun 12;4(1):e254.
- Takamaru H, Saito Y, Toyoshima N, Yamada M, Sakamoto T, Matsuda T. Polyglycolic acid sheet with clipping for closing delayed perforation after colonic endoscopic submucosal dissection. Endoscopy. 2023 Dec;55(S 01):E211-E213.
- Saito Y, Sakamoto T, Dekker E, Pioche M, Probst A, Ponchon T, Messmann H, Dinis-Ribeiro M, Matsuda T, Ikematsu H, Saito S, Wada Y, Oka S, Sano Y, Fujishiro M, Murakami Y, Ishikawa H, Inoue H, Tanaka S, Tajiri H; IEE-JNET Group. First report from the International Evaluation of Endoscopic classification Japan NBI Expert Team: International multicenter web trial. Dig Endosc. 2023 Sep 13. doi: 10.1111/den.14682. Epub ahead of print.
- Abe SK, Ihira H, Minami T, Imatoh T, Inoue Y, Tsutsumimoto K, Kobayashi N, Kashima R, Konishi M, Doi T, Teramoto M, Kabe I, Lee S, Watanabe M, Dohi S, Sakai Y, Nishita Y, Morisaki N, Tachimori H, Kokubo Y, Yamaji T, Shimada H, Mizoue T, Sawada N, Tsugane S, Iwasaki M, Inoue M. Prevalence of family history of cancer in the NC-CCAPH consortium of Japan. Sci Rep. 2023;13(1):3128.
- Ishikawa H, Yamada M, Sato Y, Tanaka S, Akiko C, Tajika M, Doyama H, Takayama T, Ohda Y, Horimatsu T, Sano Y, Tanakaya K, Ikematsu H, Saida Y, Ishida H, Takeuchi Y, Kashida H, Kiriyama S, Hori S, Lee K, Tashiro J, Kobayashi N, Nakajima T, Suzuki S, Mutoh M. Intensive endoscopic resection for downstaging of polyp burden in patients with familial adenomatous polyposis (J-FAPP Study III): a multicenter prospective interventional study.; J-FAPP Study III Group. Endoscopy. 2023 Apr;55(4):344-352.
- Kajiwara Y, Oka S, Tanaka S, Nakamura T, Saito S, Fukunaga Y, Takamatsu M, Kawachi H, Hotta K, Ikematsu H, Kojima M, Saito Y, Yamada M, Kanemitsu Y, Sekine S, Nagata S, Yamada K, Kobayashi N, Ishihara S, Saitoh Y, Matsuda K, Togashi K, Komori K, Ishiguro M, Kuwai T, Okuyama T, Ohuchi A, Ohnuma S, Sakamoto K, Sugai T, Katsumata K, Matsushita HO, Yamano HO, Eda H, Uraoka T, Akimoto N, Kobayashi H, Ajioka Y, Sugihara K, Ueno H. Nomogram as a novel predictive tool for lymph node metastasis in T1 colorectal cancer treated with endoscopic resection: a nationwide, multicenter study. Gastrointest Endosc. 2023 Jun;97(6):1119-1128.
- Kawamura T, Sekiguchi M, Takamaru H, Mizuguchi Y, Horiguchi G, Kato M, Kobayashi K, Sada M, Oda Y, Yokoyama A, Utsumi T, Tsuji Y, Ohki D, Takeuchi Y, Shichijo S, Ikematsu H, Matsuda K, Teramukai S, Kobayashi N, Matsuda T, Saito Y, Tanaka K. "Endoscopic" adenoma detection rate as a quality indicator of colonoscopy: First report from the J-SCOUT study. Dig Endosc. 2023 Jul;35(5):615-624.
- Kawamura T, Sekiguchi M, Takamaru H, Mizuguchi Y, Horiguchi G, Toyoizumi H, Kato M, Kobayashi K, Sada M, Oda Y, Yokoyama A, Utsumi T, Tsuji Y, Ohki D, Takeuchi Y, Shichijo S, Ikematsu H, Matsuda K, Teramukai S, Kobayashi N, Matsuda T, Saito Y, Tanaka K. Endoscopist-related factors affecting adenoma detection during colonoscopy: data from the J-SCOUT study. Dig Endosc.2024 Jan;36(1):51-58.
- Hihara D, Takamaru H, Sekiguchi M, Yamada M, Sakamoto T, Matsuda T, Saito Y. Factors associated with increased duration of endoscopic submucosal dissection for rectal tumors: a 22-year retrospective analysis. Gastrointest Endosc. 2023 Sep;98(3):420-427.e1.
- Saito E, Mutoh M, Ishikawa H, Kamo K, Fukui K, Hori M, Ito Y, Chen Y, Sigel B, Sekiguchi M, Hemmi O, Katanoda K. Cost-effectiveness of preventive aspirin use and intensive downstaging polypectomy in patients with familial adenomatous polyposis: A microsimulation modeling study. Cancer Med. 2023 Sep;12(18):19137-19148.
