HOME > Publication & Reports > Annual Report 2016 > Hospital East
Department of Head and Neck Medical Oncology
Makoto Tahara, Susumu Okano, Tomohiro Enokida, Yuri Ueda, Takao Fujisawa
Introduction
The Department of Head and Neck Medical Oncology is engaged in the clinical management of patients with head and neck cancer (HNC), and research into anticancer drugs for the treatment of HNC.
Our missions are to: 1) provide the best evidence-based treatment; 2) promote the importance of supportive care in the treatment of patients with HNC; 3) facilitate the timely approval of new drugs by active participation in global clinical trials to eliminate the drug lag; 4) develop cutting-edge treatments; and 5) train experts in head and neck medical oncology.
Our team and what we do
Our department consists of three physicians and two residents. We manage the treatment of HNC patients who receive anticancer drugs. An estimated 60% of HNC patients require a multidisciplinary approach, including surgery, radiotherapy, and chemotherapy. Furthermore, HNC patients are at risk of injury and impairment of vital organs both from the cancer itself and from the series of treatments provided to cure it. In treating patients, we therefore carefully assess both the curability of the condition and possible subsequent complications, such as swallowing dysfunction and cosmetic changes. Given the increasing complexity of the management of HNC, recommended treatment for patients who are referred to our institution is decided at weekly tumor board meetings attended by a multidisciplinary team.
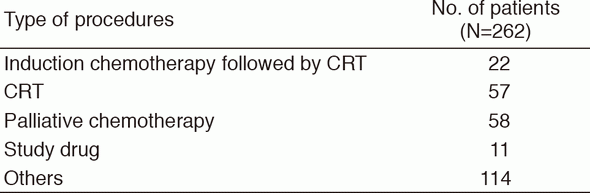
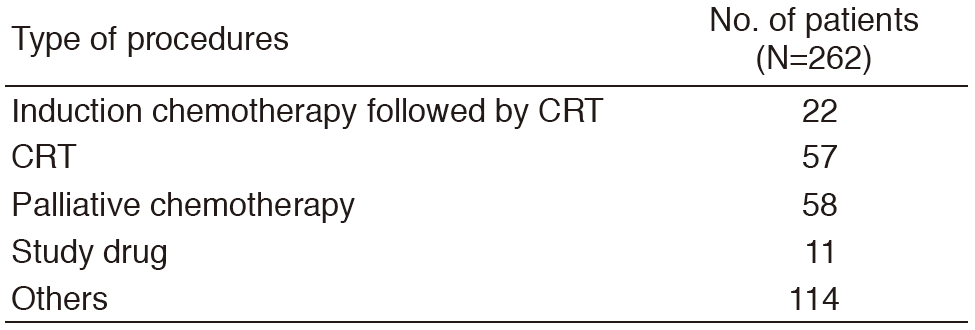
A total of 262 patients were referred to our department from January to December 2016 (Tables 1, 2). The outpatient service of our department is available from Monday to Friday. We carefully follow patients during and after treatment and provide palliative chemotherapy as an outpatient service.
Table 1. Number of patients according to sites


Table 2. Number of patients according to procedures
Research activities
Our research activity has focused on three areas, the development of new treatments in clinical trials for HNC, biomarker analysis in HNC, and retrospective analysis of the management of treatment for HNC.
1.Development of new treatments
In a phase II trial of a combination with paclitaxel, carboplatin, and cetuximab (PCE) as a first line treatment in patients with recurrent and/or metastatic SCCHN(CSPOR-HN02), PCE demonstrated promising clinical activity including overall response rate of 40%, median PFS of 5.2 months, and median OS of 14.7 months which indicates to a candidate regimen to evaluate a next phase III trial compared with standard EXTREME regimen (Tahara M et al., ASCO2016).
2.Biomarker analysis
We previously reported Cytokeratin4 (CK4) protein expression is a favorable prognostic factor in locally advanced oral tongue squamous cell carcinoma (TSCC) from cDNA microarray and immunohistocheical analysis (ASCO2015, abstract 6032). Seventy-nine primary TSCCs were examined to identify predictive markers of recurrence among clinicopathological factors and CK4 protein expression (Enokida T et al., ESMO 2016). Multivariate analysis indicated that pT [pT2 vs. pT1], tumor thickness [≥5mm vs. <5mm], lymphatic invasion [present vs. absent] and positive immunohisthochemical staining for CK4 protein of tumor cells [present vs. absent] were independent predictors for postoperative recurrence. The combination of CK4 and clinicopathlogical factor could predict risk of recurrence in this population.
3.Retrospective analysis of the management of treatment for HNC
We conducted a retrospective analysis to evaluate the efficacy and safety of cetuximab plus radiation with or without prophylactic percutaneous endoscopic gastrectomy (PEG) in locally advanced SCCHN patients who were not suitable to receive platinum (Yamazaki T et al., JJCO2016). The incidence of grade 3 or 4 mucositis tended to be higher in patients without PEG placement than in those with (83% vs. 47%). Five of twelve patients without PEG placement required interruption of treatment. More patients without PEG had significantly >10% weight loss than patients with (75% vs. 27%). Prophylactic PEG-feeding tube placement could reduce the incidence of severe toxicities, including mucositis and weight loss, and avoid RT interruption.
We retrospectively reviewed 131 head and neck cancer patients who received Cmab-containing therapy to investigate the incidence and risk factors of hypomagnesemia (Enokida T et al., Frontier in Onco 2016). Independent risk factors were low baseline serum Mg, >/=7 Cmab administrations, and concurrent administration of platinum. Cmab is associated with a significant risk of hypomagnesemia in patients with head and neck cancer with longer term administration and concurrent platinum therapy.
Clinical trials
The following investigator-initiated clinical trials are ongoing; 1) a feasibility study of a combination with paclitaxel, carboplatin, and cetuximab (PCE) as induction chemotherapy for unresectable locally advanced SCCHN, 2) JCOG1008: a randomized phase II/III trial of postoperative CRT comparing weekly CDDP plus RT with three weekly CDDP plus RT in high risk patients with SCCHN, 3) a phase II study of lenvatinib for anaplastic thyroid cancer, and 4) a cohort study exploring the effect of lenvatinib on differentiated thyroid cancer.
To facilitate the timely approval of new drugs and eliminate the drug lag, we have also participated in the global phase trials including immune-checkpoint inhibitors.
Education
We educate not only medical staff in our institute, but also outside of our institute by conducting the following education programs: 1) Seminar of Japanese Society of Supportive Care for Patients with HNC and 2) Preceptorship in HNC. A number of Asian physicians participated in preceptorship in HNC this year. Furthermore, our department is accepting trainees all the time.
Future prospects
We hope that ongoing or planned clinical trials will change the standard of care for HNC and our biomarker analysis will lead to the development of new treatment strategies. Our education programs will increase the number of medical oncologists who take charge of treatment for HNC, leading to improving patient's quality of life.
List of papers published in 2016
Journal
1.Clement PM, Gauler T, Machiels JP, Haddad RI, Fayette J, Licitra LF, Tahara M, Cohen EEW, Cupissol D, Grau JJ, Guigay J, Caponigro F, de Castro G, Jr., de Souza Viana L, Keilholz U, Del Campo JM, Cong XJ, Ehrnrooth E, Vermorken JB. Afatinib versus methotrexate in older patients with second-line recurrent and/or metastatic head and neck squamous cell carcinoma: subgroup analysis of the LUX-Head & Neck 1 trial. Ann Oncol, 27:1585-1593, 2016
2.Enokida T, Suzuki S, Wakasugi T, Yamazaki T, Okano S, Tahara M. Incidence and Risk Factors of Hypomagnesemia in Head and Neck Cancer Patients Treated with Cetuximab. Front Oncol, 6:196, 2016
3.Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med, 375:1856-1867, 2016
4.Licitra L, Keilholz U, Tahara M, Lin J-C, Chomette P, Ceruse P, Harrington K, Mesia R. Evaluation of the benefit and use of multidisciplinary teams in the treatment of head and neck cancer. Oral Oncol, 59:73-79, 2016
5.Robinson B, Schlumberger M, Wirth LJ, Dutcus CE, Song J, Taylor MH, Kim S-B, Krzyzanowska MK, Capdevila J, Sherman SI, Tahara M. Characterization of Tumor Size Changes Over Time From the Phase 3 Study of Lenvatinib in Thyroid Cancer. J Clin Endocrinol Metab, 101:4103-4109, 2016
6.Satake H, Tahara M, Mochizuki S, Kato K, Hara H, Yokota T, Kiyota N, Kii T, Chin K, Zenda S, Kojima T, Bando H, Yamazaki T, Iwasa S, Honma Y, Hamauchi S, Tsushima T, Ohtsu A. A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol, 78:91-99, 2016
7.Ooishi M, Motegi A, Kawashima M, Arahira S, Zenda S, Nakamura N, Ariji T, Tokumaru S, Sakuraba M, Tahara M, Hayashi R, Akimoto T. Patterns of failure after postoperative intensity-modulated radiotherapy for locally advanced and recurrent head and neck cancer. Jpn J Clin Oncol, 46:919-927, 2016
8.Yamazaki T, Enokida T, Wakasugi T, Zenda S, Motegi A, Arahira S, Akimoto T, Tahara M. Impact of prophylactic percutaneous endoscopic gastrostomy tube placement on treatment tolerance in head and neck cancer patients treated with cetuximab plus radiation. Jpn J Clin Oncol, 46:825-831, 2016
9.Motegi A, Fujii S, Zenda S, Arahira S, Tahara M, Hayashi R, Akimoto T. Impact of Expression of CD44, a Cancer Stem Cell Marker, on the Treatment Outcomes of Intensity Modulated Radiation Therapy in Patients With Oropharyngeal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys, 94:461-468, 2016
10.Enokida T, Fujii S, Kuno H, Mukaigawa T, Tahara M, Sakuraba M, Hayashi R. Combined salivary duct carcinoma and squamous cell carcinoma suspected of carcinoma ex pleomorphic adenoma. Pathol Int, 66:460-465, 2016
