HOME > Publication & Reports > Annual Report 2016 > Hospital East
Department of Thoracic Oncology
Koichi Goto, Seiji Niho, Kiyotaka Yoh, Shigeki Umemura, Shingo Matsumoto, Keisuke Kirita, Hibiki Udagawa, Eri Sugiyama, Yoshitaka Zenke, Masayuki Ishibashi, Koichi Saruwatari, Kakeru Hisakane, Reiko Matsuzawa, Yuko Usui, Takahiro Ohta
Introduction
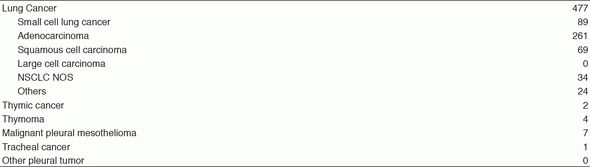
The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. Our department aims to provide the highest quality treatment and establish new effective treatments against lung cancer and other thoracic malignancies through innovative clinical and translational research. To provide assistance to our patients through multidisciplinary care, the staff members of our department work closely with thoracic surgeons, radiation oncologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.
Our team and what we do
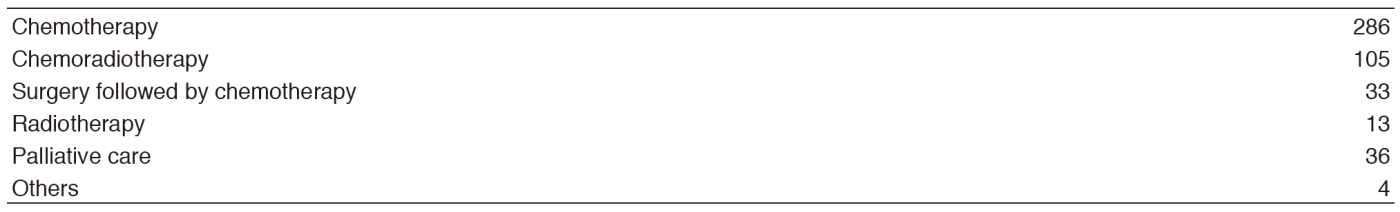
Our Outpatient Clinic, managed by the staff members and senior residents, is open from Monday to Friday for the examination of all new referred patients and the evaluation of returning patients. Returning patients also receive oral chemotherapy and/or intravenous chemotherapy in the Ambulatory Care Center. Bronchoscopy and EBUS for diagnosis is performed on Monday, Tuesday, and Thursday afternoon. Fluoroscopic-CT guided needle lung biopsies are carried out on Tuesday afternoon. For patient management, we use approximately 70 beds in 8F, 6A, 5A, and 5B wards.
Case conferences on thoracic surgery and medical oncology are scheduled on Tuesday evenings and Wednesday evenings, respectively. The staff members and residents of our department participate in a journal club on Monday and Wednesday mornings. At monthly meetings with physicians in private practice, the staff members and residents are teaching methods for reading chest X-ray and CT scan films.
Research activities
Our research activities are focused on four areas: 1) development of new and effective diagnosis and treatment modalities; 2) detection, diagnosis, and treatment of peripheral-type minute lung cancers that are not visible in plain chest X-rays; 3) collaborative studies with the Exploratory Oncology Research & Clinical Trial Center (NCC-EPOC) in the following areas: detection of driver mutation for small cell lung cancer; the development of a new diagnostic method of rare driver gene alterations for lung cancer; correlation between gene abnormalities and clinical characteristics; correlation between sensitivity of EGFR-TKI and CAF (cancer-associated fibroblasts); and 4) translational research from bench to bed-side or from bed-side to bench for the development of innovative treatment strategies.
Especially, whole genome analysis of small cell cancer to detect new driver mutations and establishment of multiplex diagnosis methods for rare gene alteration of lung cancer such as ALK, RET and ROS1 fusion gene, and BRAF mutation are under investigation as a collaboration with the NCC-EPOC.
Clinical trials
The Department of Thoracic Oncology is currently conducting and participating in multi-institutional phase III studies to establish new standard treatments against lung cancer such as the Japan Clinical Oncology Group (JCOG) trials, West Japan Oncology Group (WJOG), Thoracic Oncology Research Group (TORG) and global trials conducted by pharmaceutical companies.
Recently, the usefulness of TS-1 and pemetrexed combined with thoracic radiotherapy has been reported for locally advanced non-small-cell lung cancer (NSCLC). Therfore, the randomized phase II study of cisplatin plus TS-1 vs. cisplatin plus pemetrexed combined with thoracic radiotherapy for stage III non-squamous NSCLC was initiated in January 2013 and the patient enrollment was completed in October 2016.
Alectinib is a newly developing selective ALK inhibitor and very effective for ALK fusion positive NSCLC, although 4-5% of NSCLC are positive for ALK fusion protein. A phase III study of alectinib comparing with crizotinib for ALK positive lung cancer was completed and the results was presented at the 2016 ASCO annual meeting. The study of AZD9291, 3rd generation EGFR-TKI, was conducted and a good response for T790M-resistant mutation positive lung cancer was observed with minimal toxicities.
In addition, many recent clinical trials indicated that PD-1/PD-L1 immune checkpoint inhibitors showed remarkable clinical response against advanced NSCLC including squamous cell lung cancer. Nivolumab and pembrolizumab were approved in Japan for patients with advanced NSCLC as first-line and second-line treatment.
LC-SCRUM-Japan (Lung Cancer Genomic Screening Project for Individualized Medicine in Japan), a nation wide genomic screening project of lung cancer with rare driver oncogenes, such as ALK, RET and ROS1 fusion, and BRAF mutation was started in February 2013. As of March 2017, 4,118 patients were enrolled and 90 (3%) RET and 140 (4%) ROS1 fusion positive patients were detected. Many lung cancers with oncogenic alterations detected in LC-SCRUM-Japan had been entered into clinical trials of molecular targeting agents. In addition, from February 2015, to further develop genomic screening and to establish precision medicine in Japan, LC-SCRUM-Japan and genomic screening network for gastrointestinal cancer (GI-SCREEN), developed the collaborative genomic screening organization between academia and 16 pharmaceutical companies, named SCRUM-Japan.
List of papers published in 2016
Journal
1.Goto K, Endo M, Kusumoto M, Yamamoto N, Ohe Y, Shimizu A, Fukuoka M. Bevacizumab for non-small-cell lung cancer: A nested case control study of risk factors for hemoptysis. Cancer Sci, 107:1837-1842, 2016
2.Goto K, Ohe Y, Shibata T, Seto T, Takahashi T, Nakagawa K, Tanaka H, Takeda K, Nishio M, Mori K, Satouchi M, Hida T, Yoshimura N, Kozuki T, Imamura F, Kiura K, Okamoto H, Sawa T, Tamura T. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol, 17:1147-1157, 2016
3.Shiraishi K, Okada Y, Takahashi A, Kamatani Y, Momozawa Y, Ashikawa K, Kunitoh H, Matsumoto S, Takano A, Shimizu K, Goto A, Tsuta K, Watanabe S, Ohe Y, Watanabe Y, Goto Y, Nokihara H, Furuta K, Yoshida A, Goto K, Hishida T, Tsuboi M, Tsuchihara K, Miyagi Y, Nakayama H, Yokose T, Tanaka K, Nagashima T, Ohtaki Y, Maeda D, Imai K, Minamiya Y, Sakamoto H, Saito A, Shimada Y, Sunami K, Saito M, Inazawa J, Nakamura Y, Yoshida T, Yokota J, Matsuda F, Matsuo K, Daigo Y, Kubo M, Kohno T. Association of variations in HLA class II and other loci with susceptibility to EGFR-mutated lung adenocarcinoma. Nat Commun, 7:12451, 2016
4.Kakinuma R, Noguchi M, Ashizawa K, Kuriyama K, Maeshima AM, Koizumi N, Kondo T, Matsuguma H, Nitta N, Ohmatsu H, Okami J, Suehisa H, Yamaji T, Kodama K, Mori K, Yamada K, Matsuno Y, Murayama S, Murata K. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol, 11:1012-1028, 2016
5.Scagliotti G, Nishio M, Satouchi M, Valmadre G, Niho S, Galetta D, Cortinovis D, Benedetti F, Yoshihara E, Makris L, Inoue A, Kubota K. A phase 2 randomized study of TAS-102 versus topotecan or amrubicin in patients requiring second-line chemotherapy for small cell lung cancer refractory or sensitive to frontline platinum-based chemotherapy. Lung Cancer, 100:20-23, 2016
6.Atagi S, Goto K, Seto T, Yamamoto N, Tamura T, Tajima K, Inagaki N. Erlotinib for Japanese patients with activating EGFR mutation-positive non-small-cell lung cancer: combined analyses from two Phase II studies. Future Oncol, 12:2117-2126, 2016
7.Suzuki K, Yamanaka T, Hashimoto H, Shimada Y, Arata K, Matsui R, Goto K, Takiguchi T, Ohyanagi F, Kogure Y, Nogami N, Nakao M, Takeda K, Azuma K, Nagase S, Hayashi T, Fujiwara K, Shimada T, Seki N, Yamamoto N. Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol, 27:1601-1606, 2016
8.Bunn PA, Jr., Minna JD, Augustyn A, Gazdar AF, Ouadah Y, Krasnow MA, Berns A, Brambilla E, Rekhtman N, Massion PP, Niederst M, Peifer M, Yokota J, Govindan R, Poirier JT, Byers LA, Wynes MW, McFadden DG, MacPherson D, Hann CL, Farago AF, Dive C, Teicher BA, Peacock CD, Johnson JE, Cobb MH, Wendel H-G, Spigel D, Sage J, Yang P, Pietanza MC, Krug LM, Heymach J, Ujhazy P, Zhou C, Goto K, Dowlati A, Christensen CL, Park K, Einhorn LH, Edelman MJ, Giaccone G, Gerber DE, Salgia R, Owonikoko T, Malik S, Karachaliou N, Gandara DR, Slotman BJ, Blackhall F, Goss G, Thomas R, Rudin CM, Hirsch FR. Small Cell Lung Cancer: Can Recent Advances in Biology and Molecular Biology Be Translated into Improved Outcomes? J Thorac Oncol, 11:453-474, 2016
9.Kirita K, Izumo T, Matsumoto Y, Hiraishi Y, Tsuchida T. Bronchoscopic Re-biopsy for Mutational Analysis of Non-small Cell Lung Cancer. Lung, 194:371-378, 2016
10.Sekine I, Sumi M, Satouchi M, Tsujino K, Nishio M, Kozuka T, Niho S, Nihei K, Yamamoto N, Harada H, Ishikura S, Tamura T. Feasibility study of chemoradiotherapy followed by amrubicin and cisplatin for limited-disease small cell lung cancer. Cancer Sci, 107:315-319, 2016
11.Nishio M, Goto K, Chikamori K, Hida T, Katakami N, Maemondo M, Ohishi N, Tamura T. Analysis of Epidermal Growth Factor Receptor Mutations in Serum Among Japanese Patients Treated With First-Line Erlotinib for Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer, 17:24-29 e21, 2016
12.Doi T, Lee K-H, Kim T-M, Ohtsu A, Kim TY, Ikeda M, Yoh K, Gallo Stampino C, Hirohashi T, Suzuki A, Fujii Y, Andrew Williams J, Bang Y-J. A phase I study of the human anti-activin receptor-like kinase 1 antibody PF-03446962 in Asian patients with advanced solid tumors. Cancer Med, 5:1454-1463, 2016
13.Yoh K, Doi T, Ohmatsu H, Kojima T, Takahashi H, Zenke Y, Wacheck V, Enatsu S, Nakamura T, Turner K, Uenaka K. A phase I dose-escalation study of LY2875358, a bivalent MET antibody, given as monotherapy or in combination with erlotinib or gefitinib in Japanese patients with advanced malignancies. Invest New Drugs, 34:584-595, 2016
14.Yoh K, Hosomi Y, Kasahara K, Yamada K, Takahashi T, Yamamoto N, Nishio M, Ohe Y, Koue T, Nakamura T, Enatsu S, Lee P, Ferry D, Tamura T, Nakagawa K. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer, 99:186-193, 2016
15.Yoshino T, Kojima T, Bando H, Yamazaki T, Naito Y, Mukai H, Fuse N, Goto K, Ito Y, Doi T, Ohtsu A. Effect of food on the pharmacokinetics of TAS-102 and its efficacy and safety in patients with advanced solid tumors. Cancer Sci, 107:659-665, 2016
16.Saruwatari K, Umemura S, Nomura S, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Ohe Y, Goto K. Prognostic Factor Analysis in Patients With Small-Cell Lung Cancer Treated With Third-Line Chemotherapy. Clin Lung Cancer, 17:581-587, 2016
17.Zenke Y, Umemura S, Sugiyama E, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Goto K. Successful treatment with afatinib after grade 3 hepatotoxicity induced by both gefitinib and erlotinib in EGFR mutation-positive non-small cell lung cancer. Lung Cancer, 99:1-3, 2016
18.Zenke Y, Umemura S, Motegi A, Furukawa K, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Tsuboi M, Akimoto T, Goto K. Acute and Progressive Tracheal Stenosis after Proton Beam Therapy with Concurrent Chemotherapy for Non-Small Cell Lung Cancer. J Thorac Oncol, 11:1181-1183, 2016
19.Zenke Y, Yoh K, Matsumoto S, Umemura S, Niho S, Ohmatsu H, Goto K, Ohe Y. Clinical Impact of Gastric Acid-Suppressing Medication Use on the Efficacy of Erlotinib and Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer Harboring EGFR Mutations. Clin Lung Cancer, 17:412-418, 2016
20.Tamiya M, Tamiya A, Kaneda H, Nakagawa K, Yoh K, Goto K, Okamoto H, Shimokawa T, Abe T, Tanaka H, Daga H, Takeda K, Hirashima T, Atagi S. A phase II study of pemetrexed plus carboplatin followed by maintenance pemetrexed as first-line chemotherapy for elderly patients with advanced non-squamous non-small cell lung cancer. Med Oncol, 33:2, 2016
21.Niho S, Nokihara H, Nihei K, Akimoto T, Sumi M, Ito Y, Yoh K, Goto K, Ohmatsu H, Horinouchi H, Yamamoto N, Sekine I, Kubota K, Ohe Y, Tamura T. Dose-Escalation Study of Thoracic Radiotherapy in Combination With Pemetrexed Plus Cisplatin in Japanese Patients With Locally Advanced Nonsquamous Non-Small Cell Lung Cancer: A Post Hoc Analysis of Survival and Recurrent Sites. Am J Clin Oncol, 39:132-135, 2016
22.Neri S, Hashimoto H, Kii H, Watanabe H, Masutomi K, Kuwata T, Date H, Tsuboi M, Goto K, Ochiai A, Ishii G. Cancer cell invasion driven by extracellular matrix remodeling is dependent on the properties of cancer-associated fibroblasts. J Cancer Res Clin Oncol, 142:437-446, 2016
23.Hisakane K, Saruwatari K, Fujii S, Kirita K, Umemura S, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Kuwata T, Ochiai A, Gemma A, Tsuboi M, Goto K, Ishii G. Unique intravascular tumor microenvironment predicting recurrence of lung squamous cell carcinoma. J Cancer Res Clin Oncol, 142:593-600, 2016
24.Saruwatari K, Ikemura S, Sekihara K, Kuwata T, Fujii S, Umemura S, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Ochiai A, Kohrogi H, Tsuboi M, Goto K, Ishii G. Aggressive tumor microenvironment of solid predominant lung adenocarcinoma subtype harboring with epidermal growth factor receptor mutations. Lung Cancer, 91:7-14, 2016
25.Matsuzawa R, Kirita K, Kuwata T, Umemura S, Matsumoto S, Fujii S, Yoh K, Kojima M, Niho S, Ohmatsu H, Ochiai A, Tsuboi M, Goto K, Ishii G. Factors influencing the concordance of histological subtype diagnosis from biopsy and resected specimens of lung adenocarcinoma. Lung Cancer, 94:1-6, 2016
26.Hata K, Yoshida J, Udagawa H, Hashimoto H, Fujii S, Hishida T, Kuwata T, Aokage K, Kojima M, Ochiai A, Suzuki K, Tsuboi M, Ishii G. The difference in Ezrin-pAkt signaling axis between lepidic and papillary predominant invasive adenocarcinomas of the lung. J Cancer Res Clin Oncol, 142:1421-1430, 2016