HOME > Publication & Reports > Annual Report 2016 > Hospital
Department of Esophageal Surgery
Yuji Tachimori, Hiroyasu Igaki, Kazuo Koyanagi, Jun Iwabu, Fumihiko Kato
Introduction
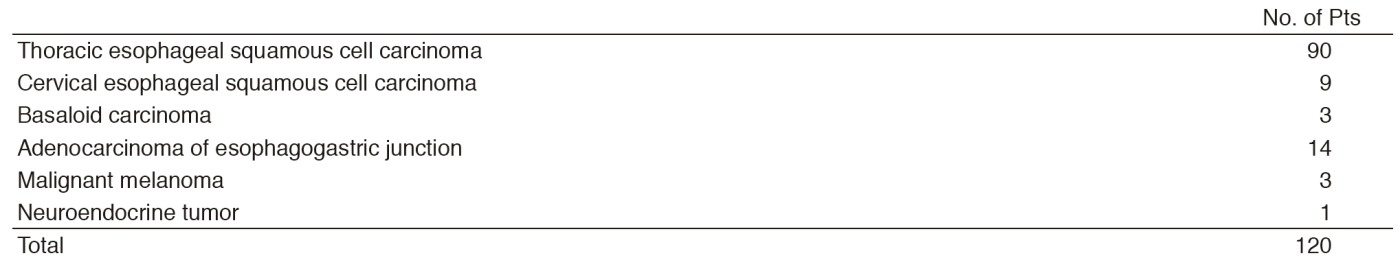
More than 300 new patients with esophageal tumors are admitted to the National Cancer Center Hospital (NCCH) every year. The multidisciplinary treatment plans are determined by the stage of the tumor in close cooperation with other teams. The Department of Esophageal Surgery particularly cooperates with the Department of Gastrointestinal Medical Oncology and the Department of Radiation Oncology for preoperative chemotherapy and chemoradiotherapy and salvage surgery after definitive chemoradiotherapy, and with the Department of Endoscopy for diagnosis and endoscopic resection. We also maintain close cooperation with the Department of Head and Neck Oncology for cervical esophageal carcinomas and with the Department of Gastric Surgery for adenocarcinomas in the esophagogastric junction. Patients who required a laryngectomy for resection of cervical esophageal cancer were operated with the Department of Head and Neck Oncology. Most patients with Siewert Type III adenocarcinoma were operated on in the Department of Gastric Surgery. In our department, squamous cell carcinomas still constitute the largest proportion of esophageal tumors, and 14 patients with adenocarcinomas of esophagogastric junction underwent an esophagectomy in 2016.
Our team and what we do
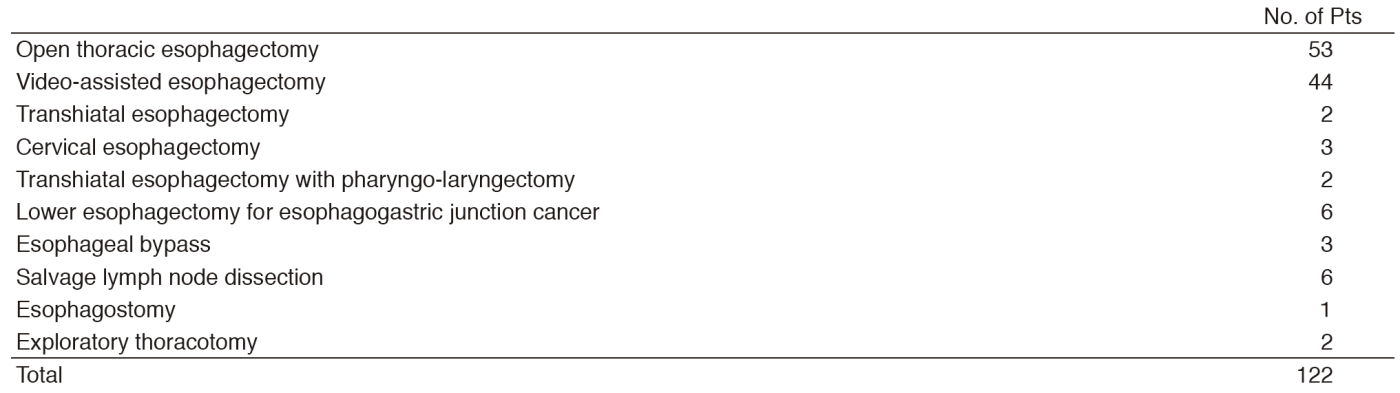
The Department of Esophageal Surgery consists of three staff surgeons, one chief resident, and one to two rotating senior residents. A multidisciplinary conference (Esophageal Tumor Board) is held weekly in which surgeons, medical oncologists, radiation oncologists, endoscopists, radiologists, and pathologists who are involved in the treatment of esophageal diseases meet and discuss the diagnosis, staging, and treatment plans for patients with esophageal tumors. Every week, two to three patients with esophageal cancer undergo surgery. One hundred and twenty patients underwent esophagectomy including nine patients with cervical esophageal cancer, three with basoloid carcinoma, three with malignant melanoma, and one with neuroendocrine tumor. Preoperative chemotherapy was recommended for 59 patients and preoperative chemoradiotherapy was recommended for five patients with resectable Stage II-IV esophageal squamous cell cancer. A three-field dissection, including the whole upper mediastinum and supraclavicular area in addition to the lower mediastinum and abdomen, was performed on 64 patients as our standard procedure. Video-assisted thoracic surgery was introduced for esophagectomy as minimally invasive surgery in 44 patients. One hospital death occurred in 2016.
In a paradigm shift toward organ-sparing therapy, the number of patients who receive definitive chemoradiotherapy as their primary treatment for resectable tumors is increasing. A persistent or recurrent loco-regional disease is not infrequent after definitive chemoradiotherapy. Twenty two patients underwent salvage esophagectomy after the failure of definitive chemoradiotherapy in 2016. A three-field dissection is avoided for salvage esophagectomy.
Research activities
Several translational studies are being carried out in cooperation with the National Cancer Center Research Institute. A study of DNA methylation in biopsied specimens is also ongoing to estimate the efficacy of preoperative chemotherapy in patients with advanced esophageal cancer. Serum miRNA is being investigated as the predictive marker in patients with esophageal squamous cell carcinoma.
Clinical trials
A multi-institutional randomized controlled trial comparing standard preoperative chemotherapy (5FU and cisplatin), an intensive one (5FU and cisplatin plus docetaxel), and preoperative chemoradiotherapy (5FU and cisplatin plus 41.4 Gy irradiation) for Stage II-III esophageal cancer (JCOG 1109) is ongoing. A new multi-institutional randomized controlled trial comparing minimally invasive esophagectomy versus open thoracic esophagectomy (JCOG 1409) started registration in 2015. A Phase II trial for definitive chemoradiotherapy with or without salvage esophagectomy (JCOG 0909) has finished registration. A new Phase II trial for tri-modality strategy with docetaxel plus 5FU and cisplatin (DCF) induction chemotherapy for locally advanced unresectable esophageal cancer followed by conversion surgery for responders and chemoradiotherapy for non-responders (COSMOS) launched in 2013 and has finished registration.
Education
We accepted many surgeons from foreign countries. A dramatic increase in the incidence of adenocarcinoma has been seen in Western patients. While squamous cell carcinoma remains the predominant type of esophageal cancer in Asian patients, including Japanese patients, Japanese strategies and surgical techniques for esophageal squamous cell carcinoma are instructive for surgeons all over the world.
Future prospects
Preoperative chemotherapy and chemoradiotherapy are performed as the standard treatment for patients with Stage II/III. Because patients who wish to preserve organs are increasing, we will cooperate more closely with the Gastrointestinal Endoscopy Division in the Department of Endoscopy, the Department of Gastrointestinal Medical Oncology, and the Department of Radiation Oncology to promote the multidisciplinary treatment. Furthermore, we are going to enhance the multidisciplinary medical team. Patients requiring salvage surgery for recurrence after definitive chemoradiotherapy have been increasing, and patients expecting thoracoscopic surgery as a less invasive technique have also been increasing. Now, surgery for esophageal cancer has become complicated and much more knowledge and precise procedures should be recommended for esophageal cancer surgeons. We will accept the treatment requests for difficult cases from other hospitals to meet the expectations of our hospital as the National Center.
List of papers published in 2016
Journal
1.Yokota T, Igaki H, Kato K, Tsubosa Y, Mizusawa J, Katayama H, Nakamura K, Fukuda H, Kitagawa Y. Accuracy of preoperative diagnosis of lymph node metastasis for thoracic esophageal cancer patients from JCOG9907 trial. Int J Clin Oncol, 21:283-288, 2016
2.Nakazato H, Takeshima H, Kishino T, Kubo E, Hattori N, Nakajima T, Yamashita S, Igaki H, Tachimori Y, Kuniyoshi Y, Ushijima T. Early-stage induction of SWI/SNF mutations during esophageal squamous cell carcinogenesis. PLoS One, 11:e0147372, 2016
3.Sawada G, Niida A, Uchi R, Hirata H, Shimamura T, Suzuki Y, Shiraishi Y, Chiba K, Imoto S, Takahashi Y, Iwaya T, Sudo T, Hayashi T, Takai H, Kawasaki Y, Matsukawa T, Eguchi H, Sugimachi K, Tanaka F, Suzuki H, Yamamoto K, Ishii H, Shimizu M, Yamazaki H, Yamazaki M, Tachimori Y, Kajiyama Y, Natsugoe S, Fujita H, Mafune K, Tanaka Y, Kelsell DP, Scott CA, Tsuji S, Yachida S, Shibata T, Sugano S, Doki Y, Akiyama T, Aburatani H, Ogawa S, Miyano S, Mori M, Mimori K. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology, 150:1171-1182, 2016
4.Koyanagi K, Ozawa S, Tachimori Y. Minimally invasive esophagectomy performed with the patient in a prone position: a systematic review. Surg Today, 46:275-284, 2016
5.Koyanagi K, Ozawa S, Oguma J, Kazuno A, Yamazaki Y, Ninomiya Y, Ochiai H, Tachimori Y. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: New predictive evaluation of anastomotic leakage after esophagectomy. Medicine (Baltimore), 95:e4386, 2016
6.Tachimori Y, Ozawa S, Numasaki H, Fujishiro M, Matsubara H, Oyama T, Shinoda M, Toh Y, Udagawa H, Uno T. Comprehensive Registry of Esophageal Cancer in Japan, 2009. Esophagus, 13:110-137, 2016
7.Tachimori Y, Ozawa S, Numasaki H, Matsubara H, Shinoda M, Toh Y, Udagawa H, Fujishiro M, Oyama T, Uno T. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus, 13:1-7, 2016
8.Kataoka K, Takeuchi H, Mizusawa J, Ando M, Tsubosa Y, Koyanagi K, Daiko H, Matsuda S, Nakamura K, Kato K, Kitagawa Y. A randomized Phase III trial of thoracoscopic versus open esophagectomy for thoracic esophageal cancer: Japan Clinical Oncology Group Study JCOG1409. Jpn J Clin Oncol, 46:174-177, 2016
9.Akutsu Y, Kato K, Igaki H, Ito Y, Nozaki I, Daiko H, Yano M, Udagawa H, Nakagawa S, Takagi M, Mizusawa J, Kitagawa Y. The Prevalence of Overall and Initial Lymph Node Metastases in Clinical T1N0 Thoracic Esophageal Cancer: From the Results of JCOG0502, a Prospective Multicenter Study. Ann Surg, 264:1009-1015, 2016
10.Hamamoto Y, Mizusawa J, Katayama H, Nakamura K, Kato K, Tsubosa Y, Ishikura S, Igaki H, Shinoda M, Fukuda H, Kitagawa Y, Ando N. Inter-institutional survival heterogeneity in chemoradiation therapy for esophageal cancer: exploratory analysis of the JCOG0303 study. Jpn J Clin Oncol, 46:389-392, 2016
11.Tsushima T, Mizusawa J, Sudo K, Honma Y, Kato K, Igaki H, Tsubosa Y, Shinoda M, Nakamura K, Fukuda H, Kitagawa Y. Risk Factors for Esophageal Fistula Associated With Chemoradiotherapy for Locally Advanced Unresectable Esophageal Cancer: A Supplementary Analysis of JCOG0303. Medicine (Baltimore), 95:e3699, 2016
12.Kishino T, Niwa T, Yamashita S, Takahashi T, Nakazato H, Nakajima T, Igaki H, Tachimori Y, Suzuki Y, Ushijima T. Integrated analysis of DNA methylation and mutations in esophageal squamous cell carcinoma. Mol Carcinogenesis, 55:2077-2088, 2016