HOME > Publication & Reports > Annual Report 2016 > Hospital
Supportive Care Development Center
Narikazu Boku, Emi Fujii, Naoko Inamura, Kayo Hamaguchi
Introduction
During the treatment and management of cancer patients, because there are various unmet needs which cannot be solved by each patient's attending doctor alone, it is generally recognized that taking a team approach to these problems is very important. In September 2016, the Supportive Care Development Center was opened as the base of team medicine in the National Cancer Center Hospital.
Our team and what we do
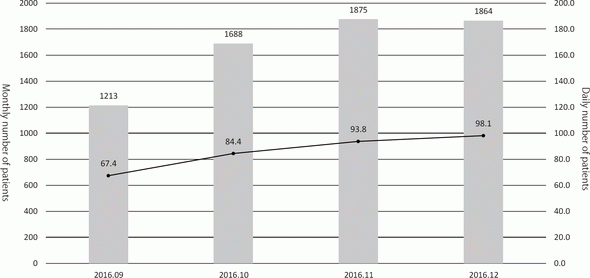
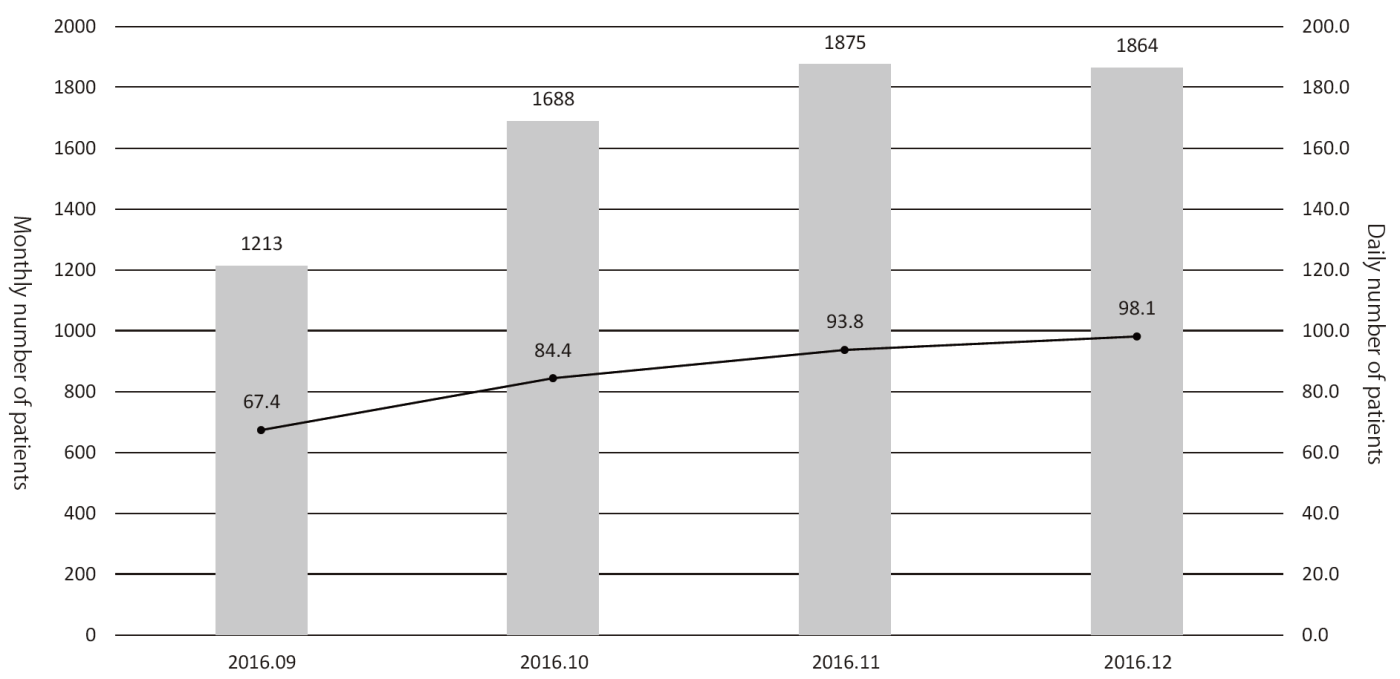
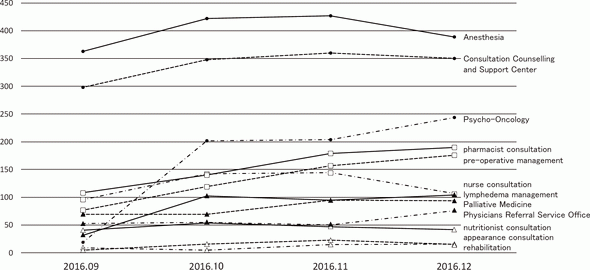
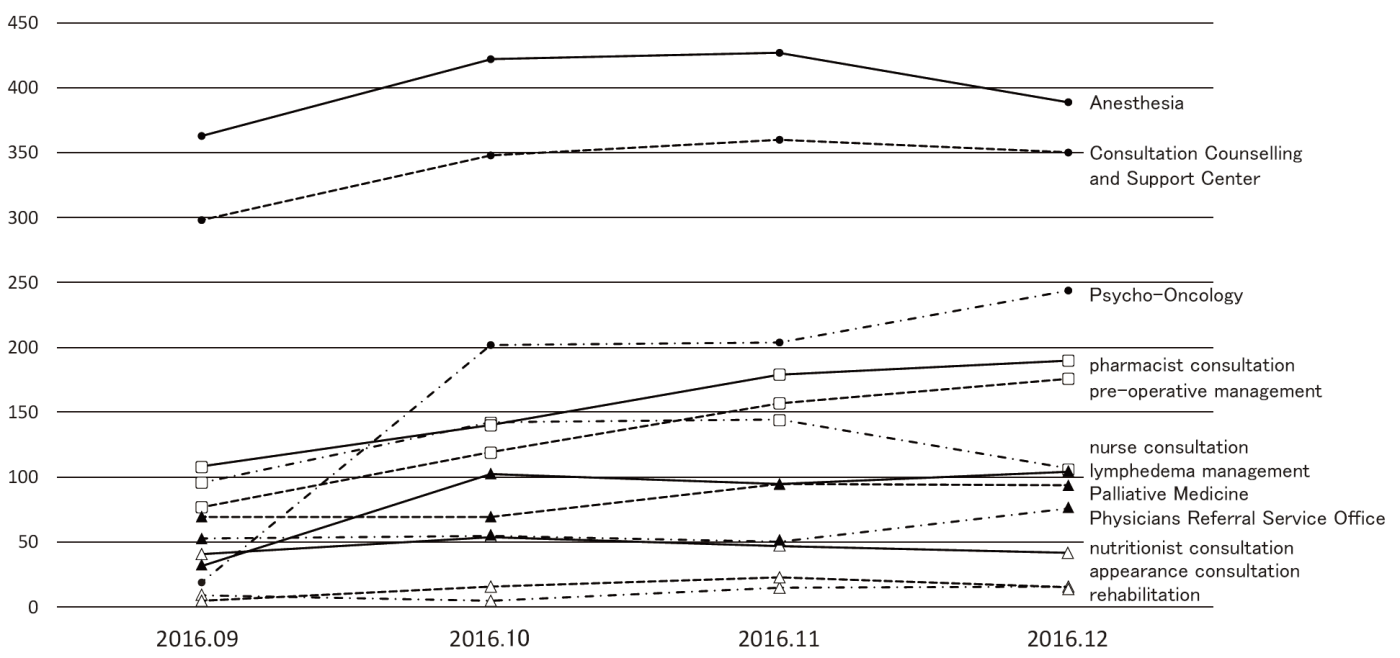
While pre-existing programs had been conducted after searching available rooms of the out-patient clinic, designated rooms for each program are prepared in the Supportive Care Development Center. There are seven standing programs such as nurse consultation, pharmacist consultation, nutritionist consultation, rehabilitation, pre-operative management, appearance consultation, and lymphedema management, for which reservation can be taken by any medical staff who find patient's unmet needs without doctor's permission. Other facilities such as the Consultation, Counseling and Support Service Center, the Physician Referral Service Office, and outpatient clinic of the Departments of Palliative Medicine, Psycho-Oncology, and Anesthesia and Intensive Care have been moved to the Supportive Care Development Center for facilitating the interaction within the team medicine. Besides the reserved patients, any patient can visit and consult about his/her unmet needs. Furthermore, there are many lectures for cancer patients and their families. After opening, the number of patients has been increasing to about 100 per day (Figure 1), and the activity of each program is also increasing (Figure 2).
We developed the questionnaire asking for patient's physical and mental pain, and pain screening of daily new patients before seeing doctors has been begun as a new program from the opening of the Supportive Care Development Center. Nurses of the out-patients clinic firstly triage patients whose physical pain > 3 points and/or mental pain > 6 points, and introduce them to the nurse consultation at the Supportive Care Development Center if necessary.
Research activities
As a result of pain screening, only 40% of patients whose physical pain > 3 points and/or mental pain > 6 points actually visited the Supportive Care Development Center. Sixty percent of patients visiting the Supportive Care Development Center were female and their most common problems were anxiety, especially about surgery. The reasons for the smaller proportion of consulting patients than expected are considered as follows.
1)Main problem such as anxiety was resolved after meeting the doctors.
2)No time to visit the Supportive Care Development Center after long waiting at the out-patient clinic.
We are now discussing about revising the procedure of pain screening. On the other hand, there were not a few patients without reservation, and their unmet needs were mainly related to adverse events of anti-cancer drugs and post-surgery symptoms.
Clinical trials
We are now preparing a protocol for the clinical study evaluating each program at the Supportive Care Development Center.
Education
Three nurses from three hospital wards participate in the nurse consultation and pre-operative management programs and receive on-the-job training under the supervision of the staff of the Supportive Care Development Center.
Future prospects
For each ongoing program, we will prepare a standard operation procedure and evaluate the outcome and spending time (cost). Considering the PDCA (plan-do-check-act) cycle method, we try to improve and newly develop our programs continuously and to be a model for other institutions taking care of cancer patients in Japan.
List of papers published in 2016
Journal
1.Akizuki N, Shimizu K, Asai M, Nakano T, Okusaka T, Shimada K, Inoguchi H, Inagaki M, Fujimori M, Akechi T, Uchitomi Y. Prevalence and predictive factors of depression and anxiety in patients with pancreatic cancer: a longitudinal study. Jpn J Clin Oncol, 46:71-77, 2016
2.Takeuchi H, Saeki T, Aiba K, Tamura K, Aogi K, Eguchi K, Okita K, Kagami Y, Tanaka R, Nakagawa K, Fujii H, Boku N, Wada M, Akechi T, Udagawa Y, Okawa Y, Onozawa Y, Sasaki H, Shima Y, Shimoyama N, Takeda M, Nishidate T, Yamamoto A, Ikeda T, Hirata K. Japanese Society of Clinical Oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary. Int J Clin Oncol, 21:1-12, 2016
3.Tabuse H, Kashiwagi H, Hamauchi S, Tsushima T, Todaka A, Yokota T, Machida N, Yamazaki K, Fukutomi A, Onozawa Y, Mori K, Boku N, Ichinose M, Yasui H. Excessive watering eyes in gastric cancer patients receiving S-1 chemotherapy. Gastric Cancer, 19:894-901, 2016
4.Tsuda T, Kyomori C, Mizukami T, Taniyama T, Izawa N, Horie Y, Hirakawa M, Ogura T, Nakajima TE, Tsugawa K, Boku N. Infusion site adverse events in breast cancer patients receiving highly emetic chemotherapy with prophylactic anti-emetic treatment with aprepitant and fosaprepitant: A retrospective comparison. Mol Clin Oncol, 4:603-606, 2016
5.Zenda S, Ota Y, Tachibana H, Ogawa H, Ishii S, Hashiguchi C, Akimoto T, Ohe Y, Uchitomi Y. A prospective picture collection study for a grading atlas of radiation dermatitis for clinical trials in head-and-neck cancer patients. J Radiat Res, 57:301-306, 2016
6.Fujiwara M, Inagaki M, Higuchi Y, Uchitomi Y, Terada S, Kodama M, Kishi Y, Yamada N. An Open-Label Feasibility Trial of Repetitive Transcranial Magnetic Stimulation for Treatment-Resistant Major Depressive Episodes. Acta Med Okayama, 70:307-311, 2016
7.Higuchi Y, Inagaki M, Koyama T, Kitamura Y, Sendo T, Fujimori M, Uchitomi Y, Yamada N. A cross-sectional study of psychological distress, burnout, and the associated risk factors in hospital pharmacists in Japan. BMC Public Health, 16:534, 2016
8.Sakamoto S, Takaki M, Okahisa Y, Mizuki Y, Inagaki M, Ujike H, Mitsuhashi T, Takao S, Ikeda M, Uchitomi Y, Iwata N, Yamada N. Individual risk alleles of susceptibility to schizophrenia are associated with poor clinical and social outcomes. J Hum Genet, 61:329-334, 2016