HOME > Publication & Reports > Annual Report 2016 > Hospital
Clinical Research Support Office
Yasuhiro Fujiwara
hClinical Trial Coordination (& Support) Office:
Noboru Yamamoto, Hiroko Nakahama, Miki Ito, Katuaki Imaizumi, Noriko Kobayashi, Kiyoka Ishihama, Katsuyuki Ikarashi, Keiko Igarashi, Yumiko Ikuno, Harue Ui, Shino Oosawa, Ran Obara, Setsuko Kamizaki, Kikue Kamiyama, Hiroko Kawaguchi, Akiko Saito, Asako Sakamoto, Ai Sekido, Yukako Takasaki, Mari Takahashi, Yuko Tanoue, Tomomi Tsuchiya, Yukiko Nishioka, Yukari Nishiyama, Kumiko Hirayama, Noriko Makita, Fumiko Mitsuishi, Hiroko Minami, Risa Miyagawa, Chie Miyano, Sho Murata, Yoshimi Yamaguchi, Tamami Yamano, Saki Yoshizawa, Katsuko Iinuma, Shiori Umino, Mai Kouda, Isoko Nakamura, Yuri Shishido, Miho Hasegawa, Tomoko Shibayama, Tsukina Soku, Ami Hashimoto, Miho Yamaguchi, Kimiko Sega, Chie Moteki
hClinical Trial Management Section:
Kenichi Nakamura, Hiroshi Katayama, Tamie Sukigara, Ritsuko Nagasaka, Tomomi Hata, Mamiko Kawasaki, Satoshi Kawashima, Eiko Yorikane, Hidekazu Saito, Akio Tsuboshita, Akane Matsushima, Makiko Watanabe, Junko Eba, Keisuke Kanato, Kenichi Miyamoto, Hideaki Kitahara, Kiyo Tanaka, Taro Shibata, Aya Kuchiba, Junki Mizusawa, Kan Yonemori, Hideki Ueno
hData Management Section
Haruhiko Fukuda, Harumi Kaba, Nobuko Okamura, Ryuji Makiuchi, Rie Nagumo, Chiho Shimojima, Yukari Hoshina, Kaoru Koike, Keiko Ohata, Keiko Suto
hBiobank and Translational Research Support Section
Ken Kato, Teiko Yamane, Suga Yamagami, Keiko Wakakuwa, Mayumi Ikeda, Haruka Sawamura, Harumi Mochizuki, Satomi Nakamori, Junko Suzuki, Keiko Shimo, Yuki Sako
Introduction
In 2015, the Research Coordination Division and the Research Promotion Division of the Center for Research Administration and Support (CRAS), the Clinical Trial Coordination (& Support) Office, and Biobank and Translational Research Support Section of the National Cancer Center Hospital (NCCH) were reorganized into the Clinical Research Support Office of the NCCH. The Clinical Research Support Office supports clinical research conducted under the leadership by investigators in the NCCH. Supporting activities include protocol writing, central/local data management, statistical design and analysis, in-house/on-site monitoring, audits, patient recruitment, and other coordinating jobs.
Our team and what we do
1.Clinical Trial Coordination (& Support) Office
The Clinical Trial Coordination (& Support) Office supports a lot of industry-sponsored registration trials as well as the physician-initiated registration-directed clinical trials. A total of 34 CRCs (clinical research coordinators) are supporting these trials.
2.Clinical Trial Management Section
The Clinical Trial Management Section has five functions: i) Multi-institutional trial support, ii) Investigational new drug (IND) trial management, iii) Biostatistics, iv) Safety management, and v) Pharmaceutical affairs consultation. One of the strengths of the NCCH is implementing various types of clinical trials covering both early phase trials including first-in-human trials and late phase multi-institutional trials. The IND trial management function is responsible for comprehensive study coordination and site visit monitoring in early phase trials. The multi-institutional trial support function works as the Japan Clinical Oncology Group (JCOG) Operations Office, which engages in protocol development, manuscript drafting, study coordination, etc., for late phase trials. The section is also responsible for the coordination of study planning consultation meetings and the concept review committee meetings.
3.Data Management Section
The Data Management Section is responsible for central data management and in-house study monitoring in the investigator-initiated clinical trials for cancer therapeutic development. This section consists of two teams; (1) Data managers in the JCOG Data Center and (2) In-house research team. The JCOG Data Center mostly supports late development multi-modality multi-institutional phase II or phase III trials for adult cancer. The in-house research team supports early phase cancer trials mainly for drug development including registration trials which are led by physicians in the NCCH.
4.Biobank and Translational Research Support Section
The Biobank and Translational Research Support Section has routinely obtained the informed consent to participate as an NCC Biobank (NCCBB) donor from patients who consult with the NCCH for the first time. Clinical research coordinators in this section coordinate translational research in several ways, such as assistance of registration for clinical trials, logistics of pathological specimens, data collection for case report forms, and the coordination between sections.
We explained the purpose of the NCCBB to 9,732 patients from January to December 2016, and received consent for blood collection and research use of their surplus samples for research from 8,886 patients (91.3% consent rate). The patient load with our assistance in filling in the preliminary-diagnosis card and so on was 10,067.
We support seven biomarker trials, and for registered patients (pts), five pts for BT-SCRUM, four pts for PRELUDE study, 27 pts for TOP-GEAR study, 155 pts for TOP-GEAR SciLabo, 28 pts for GI-SCREEN_CRC study, 87 pts for GI-SCREEN_nonCRC sudy, and 17 pts for DEF trial.
Research activities
Clinical Trial Management Section
In the academic conference, several presentations were made by the section members such as i) NCCH initiative as a Clinical Research Core Hospital, ii) Risk-based monitoring in the NCCH, and iii) Utilization of Central IRB (Institutional Review Board) in multi-institutional clinical trials.
Clinical trials
1.Clinical Trial Coordination (& Support) Office
The number of the industry-sponsored registration trials is increasing year by year, and we supported 291 registration-directed clinical trials including 24 investigator-initiated registration directed trials in 2016 (Table 1).
2.Clinical Trial Management Section
As of the end of 2016, the Clinical Trial Management Section supports 11 IND trials, two clinical trials using Advanced Medical Care system, and one registry study with umbrella-design, and the number of study planning consultations was 22 and the number of studies reviewed in the concept review committee was 18.
3.Data Management Section
The in-house research team of the section supports 12 IND trials (four open, six in preparation, and two on follow-up) and 11 non-IND trials (four open, five in preparation, and two on follow-up) including two ongoing randomized trials evaluating supportive care. The JCOG Data Center supports 94 non-IND trials (44 open, 18 in preparation, and 18 on follow-up).
Education
The staff members received not only day-to-day on-the-job training but also in-house educational seminars and the JCOG internal educational program in order to learn clinical trial methodology, project management, monitoring, and research ethics.
Future prospects
1.Clinical Trial Coordination (& Support) Office
The number of the supported clinical trials is increasing as previously described, and the supporting area covered by the CRCs will be expanded to include not only registration trials but also other investigator-initiated clinical trials. Therefore, the expansion of CRC staff members is highly anticipated. In view of the plan for the NCCH, all members of this office will work together to contribute to reinforcing the clinical research capabilities of the NCCH and to making this office a valuable unit for all members of our hospital.
2.Clinical Trial Management Section
Because the Clinical Trial Management Section has covered many functions, this section will be re-organized as the Research Management Division with six sections, including the Clinical Trial Management Section, the Project Management Section, the Multi-institutional Trial Section, the Biostatistics Section, the Regulatory Affairs Section, and the International Trial Support Section. By increasing the number of staff, we plan to develop the comprehensive clinical research support organization covering from early to late treatment development.
In 2016, the NCCH was certified as the Global Clinical Trial Core Hospital. Under this program, Asian multi-national IND trials are being prepared and a collaborative study between the JCOG and the European Organization for Research and Treatment of Cancer (EORTC) has started. As a future direction, this section will reinforce the support function for various types of clinical trials including advanced medical care system.
3.Data Management Section
This section is introducing a web-based electronic data capturing (EDC) system and is promoting the standardization of all aspects of data management, such as data format, case report forms and monitoring reports for increasing data integrity and the cost effectiveness of day-to-day work.
4.Biobank and Translational Research Support Section
Staff members of this section attended seminars relating to clinical research ethics, etc. We inform and educate investigators of the NCC about the NCC biobank and translational research periodically through in-house educational program. As a future direction, we proceed improvement of quality of the NCC biobank about informatics and storage of serum. And we aim to establish a support system with higher quality and quantity.
Table 1. Supported Trials in the Clinical Trial Coordination (& Support) Office in 2016
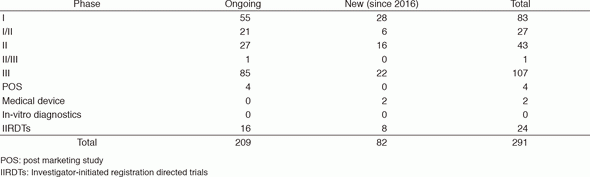
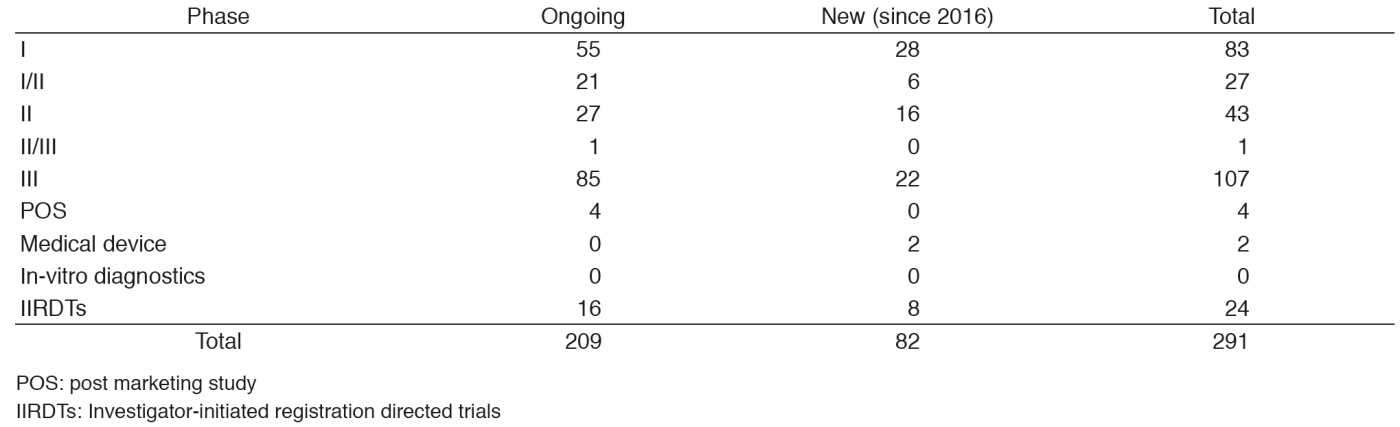
POS: post marketing study
IIRDTs: Investigator-initiated registration directed trials
List of papers published in 2016
Journal
1.Nishina T, Boku N, Gotoh M, Shimada Y, Hamamoto Y, Yasui H, Yamaguchi K, Kawai H, Nakayama N, Amagai K, Mizusawa J, Nakamura K, Shirao K, Ohtsu A. Randomized phase II study of second-line chemotherapy with the best available 5-fluorouracil regimen versus weekly administration of paclitaxel in far advanced gastric cancer with severe peritoneal metastases refractory to 5-fluorouracil-containing regimens (JCOG0407). Gastric Cancer, 19:902-910, 2016
2.Yokota T, Igaki H, Kato K, Tsubosa Y, Mizusawa J, Katayama H, Nakamura K, Fukuda H, Kitagawa Y. Accuracy of preoperative diagnosis of lymph node metastasis for thoracic esophageal cancer patients from JCOG9907 trial. Int J Clin Oncol, 21:283-288, 2016
3.Tanaka K, Hasegawa T, Nojima T, Oda Y, Mizusawa J, Fukuda H, Iwamoto Y. Prospective evaluation of Ki-67 system in histological grading of soft tissue sarcomas in the Japan Clinical Oncology Group Study JCOG0304. World J Surg Oncol, 14:110, 2016
4.Saito S, Fujita S, Mizusawa J, Kanemitsu Y, Saito N, Kinugasa Y, Akazai Y, Ota M, Ohue M, Komori K, Shiozawa M, Yamaguchi T, Akasu T, Moriya Y. Male sexual dysfunction after rectal cancer surgery: Results of a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for patients with lower rectal cancer: Japan Clinical Oncology Group Study JCOG0212. Eur J Surg Oncol, 42:1851-1858, 2016
5.Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, Wakabayashi M, Takehara K, Saito M, Ushijima K, Kobayashi H, Kawana K, Yokota H, Takano M, Takeshima N, Watanabe Y, Yaegashi N, Konishi I, Kamura T, Yoshikawa H. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer, 64:22-31, 2016
6.Nishio S, Kitagawa R, Shibata T, Yoshikawa H, Konishi I, Ushijima K, Kamura T. Prognostic factors from a randomized phase III trial of paclitaxel and carboplatin versus paclitaxel and cisplatin in metastatic or recurrent cervical cancer: Japan Clinical Oncology Group (JCOG) trial: JCOG0505-S1. Cancer Chemother Pharmacol, 78:785-790, 2016
7.Mizusawa J, Morizane C, Okusaka T, Katayama H, Ishii H, Fukuda H, Furuse J. Randomized Phase III study of gemcitabine plus S-1 versus gemcitabine plus cisplatin in advanced biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1113, FUGA-BT). Jpn J Clin Oncol, 46:385-388, 2016
8.Goto K, Ohe Y, Shibata T, Seto T, Takahashi T, Nakagawa K, Tanaka H, Takeda K, Nishio M, Mori K, Satouchi M, Hida T, Yoshimura N, Kozuki T, Imamura F, Kiura K, Okamoto H, Sawa T, Tamura T. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol, 17:1147-1157, 2016
9.Fujitani K, Yang H-K, Mizusawa J, Kim Y-W, Terashima M, Han S-U, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, Yoshikawa T, Hahn S, Nakamura K, Park CH, Kurokawa Y, Bang Y-J, Park BJ, Sasako M, Tsujinaka T. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol, 17:309-318, 2016
10.Eba J, Nakamura K, Mizusawa J, Suzuki K, Nagata Y, Koike T, Hiraoka M, Watanabe S, Ishikura S, Asamura H, Fukuda H. Stereotactic body radiotherapy versus lobectomy for operable clinical stage IA lung adenocarcinoma: comparison of survival outcomes in two clinical trials with propensity score analysis (JCOG1313-A). Jpn J Clin Oncol, 46:748-753, 2016
11.Kataoka K, Takeuchi H, Mizusawa J, Ando M, Tsubosa Y, Koyanagi K, Daiko H, Matsuda S, Nakamura K, Kato K, Kitagawa Y. A randomized Phase III trial of thoracoscopic versus open esophagectomy for thoracic esophageal cancer: Japan Clinical Oncology Group Study JCOG1409. Jpn J Clin Oncol, 46:174-177, 2016
12.Akutsu Y, Kato K, Igaki H, Ito Y, Nozaki I, Daiko H, Yano M, Udagawa H, Nakagawa S, Takagi M, Mizusawa J, Kitagawa Y. The Prevalence of Overall and Initial Lymph Node Metastases in Clinical T1N0 Thoracic Esophageal Cancer: From the Results of JCOG0502, a Prospective Multicenter Study. Ann Surg, 264:1009-1015, 2016
13.Hamamoto Y, Mizusawa J, Katayama H, Nakamura K, Kato K, Tsubosa Y, Ishikura S, Igaki H, Shinoda M, Fukuda H, Kitagawa Y, Ando N. Inter-institutional survival heterogeneity in chemoradiation therapy for esophageal cancer: exploratory analysis of the JCOG0303 study. Jpn J Clin Oncol, 46:389-392, 2016
14.Tsushima T, Mizusawa J, Sudo K, Honma Y, Kato K, Igaki H, Tsubosa Y, Shinoda M, Nakamura K, Fukuda H, Kitagawa Y. Risk Factors for Esophageal Fistula Associated With Chemoradiotherapy for Locally Advanced Unresectable Esophageal Cancer: A Supplementary Analysis of JCOG0303. Medicine (Baltimore), 95:e3699, 2016
15.Kurokawa Y, Boku N, Yamaguchi T, Ohtsu A, Mizusawa J, Nakamura K, Fukuda H. Inter-institutional heterogeneity in outcomes of chemotherapy for metastatic gastric cancer: correlative study in the JCOG9912 phase III trial. ESMO Open, 1:e000031, 2016
16.Kataoka K, Katai H, Mizusawa J, Katayama H, Nakamura K, Morita S, Yoshikawa T, Ito S, Kinoshita T, Fukagawa T, Sasako M. Non-Randomized Confirmatory Trial of Laparoscopy-Assisted Total Gastrectomy and Proximal Gastrectomy with Nodal Dissection for Clinical Stage I Gastric Cancer: Japan Clinical Oncology Group Study JCOG1401. J Gastric Cancer, 16:93-97, 2016
17.Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H, Sasako M, . Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today, 46:668-685, 2016
