HOME > Publication & Reports > Annual Report 2016 > Research Institute
Department of Omics Network
Masaru Katoh
Introduction
The Department of Omics Network is involved in innovation based on the balance between its main world-class projects and new cutting-edge projects. The WNT (PMID: 17634527), FGF (PMID: 23696246), Notch (PMID: 17143535) and Hedgehog (PMID: 19860666) signaling cascades, and the Forkhead-box (FOX) family of transcription factors (PMID: 23022474) are the main (fundamental) projects of our department. Cell adhesion (PMID 25865774), epigenetics (PMID 26411517), and microRNA (miRNA) (PMID 25364765) are new (cutting-edge) projects of our department.
Masaru Katoh was engaged in clinical medicine from 1986 to 1990, basic medicine from 1990 to 2002, and information science from 2003 to 2011. Since 2012, Masaru Katoh has been engaged in the Knowledgebase Project with the slogan "Back to the Medicine".
Research activities
1.FGF signaling-targeted therapy
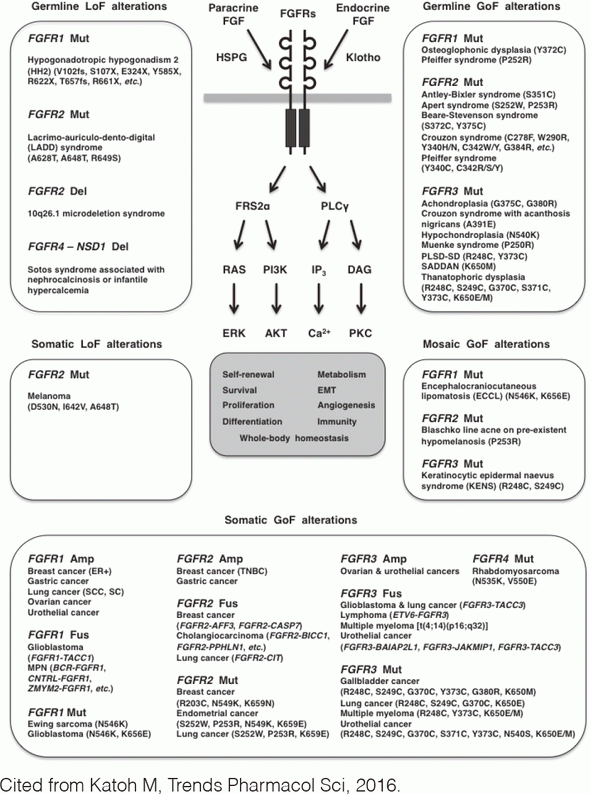
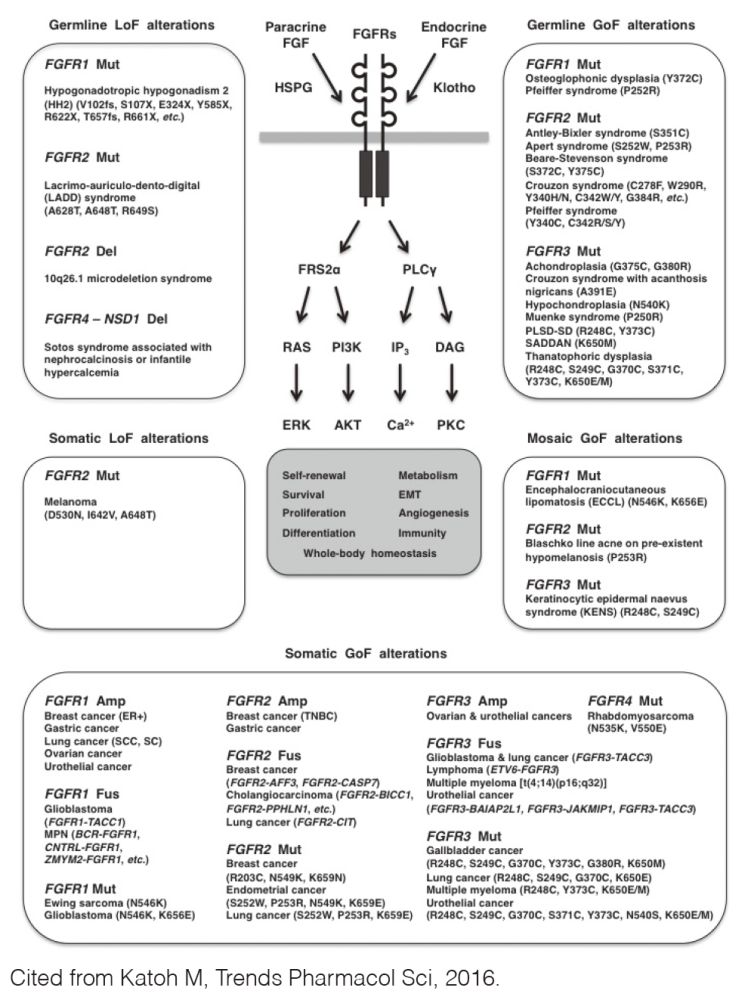
Fibroblast growth factor (FGF) signaling through FGFR1, FGFR2, FGFR3 or FGFR4 regulates cell fate, angiogenesis, immunity, and metabolism. Dysregulated FGF signaling causes human diseases, such as breast cancer, cholangiocarcinoma, chondrodysplasia, encephalocraniocutaneous lipomatosis, endometrial cancer, Ewing sarcoma, gastric cancer, glioblastoma, lung cancer, multiple myeloma, ovarian cancer, rhabdomyosarcoma, urothelial cancer, and X-linked hypophosphatemic rickets (Figure 1).
FGFR1 is amplified and overexpressed in breast and lung cancer, FGFR2 in gastric cancer and FGFR1/3 in ovarian and urothelial cancer. BCR-FGFR1, CNTRL-FGFR1, CUX1-FGFR1, FGFR1OP-FGFR1, MYO18A-FGFR1, and ZMYM2-FGFR1 fusions in myeloproliferative neoplasms are non-receptor-type FGFR kinases, whereas FGFR1-TACC1, FGFR2-AFF3, FGFR2-BICC1, FGFR2-PPHLN1, FGFR3-BAIAP2L1, and FGFR3-TACC3 fusions in solid tumors are transmembrane-type FGFRs with C-terminal alterations. N546K FGFR1, K656E FGFR1, N549K FGFR2, K659N FGFR2, N540S FGFR3, K650E/N FGFR3, and N535K FGFR4 are gain-of-function mutations, whereas D530N FGFR2, I642V FGFR2, and A648T FGFR2 are loss-of-function mutations.
Recombinant FGFs are pro-FGF signaling therapeutics for tissue and/or wound repair, whereas FGF analogs and gene therapy are under development for the treatment of cardiovascular disease, diabetes, and osteoarthritis. FGF traps, anti-FGF/FGFR monoclonal antibodies (mAbs), and small-molecule FGFR inhibitors are anti-FGF signaling therapeutics under development for the treatment of cancer, chondrodysplasia, and rickets.
FGFR kinase inhibitors were classified as FGFR1/2/3 inhibitors (AZD4547, dovitinib/TKI258, infigratinib/BGJ398, etc.), selective FGFR4 inhibitors (BLU9931) or pan-FGFR inhibitors (erdafitinib/JNJ-493/JNJ-42756493, LY2874455, ponatinib/AP24534, etc.) due to the diversification of FGFR4 compared with the other FGFR family members. By contrast, FGFR kinase inhibitors were alternatively classified as FGFR family-restricted inhibitors (erdafitinib, infigratinib, LY2874455, etc.) or FGFR/CSF1R/VEGFR family inhibitors (AZD4547, dovitinib, ponatinib, etc.) due to the evolutionary conservation among the FGFR, CSF1R, and VEGFR family members.
The tumor microenvironment consists of cancer cells and stromal/immune cells, such as cancer-associated fibroblasts (CAFs), endothelial cells, M2-type tumor-associating macrophages (M2-TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells. FGFR inhibitors elicit antitumor effects directly on cancer cells, as well as indirectly through the blockade of paracrine signaling. The dual inhibition of FGF and CSF1 or VEGF signaling is expected to enhance the antitumor effects through the targeting of immune evasion and angiogenesis in the tumor microenvironment. Combination therapies using tyrosine kinase inhibitors (FGFR or CSF1R inhibitors) and immune checkpoint blockers (anti-PD-1 or anti-CTLA-4 monoclonal antibodies) were proposed as promising choices for cancer patients in the future.
2.Epigenetics-targeted therapy
Epigenetics is currently used to describe the chromatin-dependent regulation of transcription and phenotype, while it was initially defined as heritable phenotypic changes independent of genomic alterations. Histones undergo post-translational modifications, such as methylation, acetylation, ubiquitylation, and phosphorylation. Canonical SET genes, encoding histone methyltransferases, are mutated in cancers and non-cancerous diseases. EZH2, MLL3, NSD1, NSD2, and NSD3 were identified as rational drug targets for cancer patients. GSK126 (GSK2816126) and tazemetostat (EPZ-6438) are EZH2 inhibitors that are in clinical trials for patients with B-cell lymphomas.
3.Collaborative projects
Intra-NCC collaborative study on bladder cancer genomics and international collaborative studies on FOXE1 genetics and WNT-related meta-analysis were also published in 2016.
Contribution to the global scientific community
Masaru Katoh has been contributing to the global scientific community based on manuscript publication, reviewer activity, and editor activity. Katoh carried out peer reviews of grant proposals or journal manuscripts written in English 56 times in 2016. Katoh is an Academic Editor of PLoS ONE, and carried out editorial decisions 127 times in 2016. Masaru Katoh is the Chief Editor of Frontiers in Molecular Medicine that aims to address the gap between cell and developmental biology, and clinical medicine, together with 124 editorial board members.
The manuscript citation count in the Web of Science Database (Thomson Reuters) is a surrogate marker of the contribution to the global science community. Katoh's manuscripts were cited at least 615 times by others in 2016.
List of papers published in 2016
Journal
1.Katoh M. Mutation spectra of histone methyltransferases with canonical SET domains and EZH2-targeted therapy. Epigenomics, 8:285-305, 2016
2.Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis. Int J Mol Med, 38:3-15, 2016
3.Jin J, Zhan P, Qian H, Wang X, Katoh M, Phan K, Chung JH, Lv T, Song Y; Written on behalf of the AME Lung Cancer Collaborative Group. Prognostic value of wingless-type proteins in non-small cell lung cancer patients: a meta-analysis. Transl Lung Cancer Res, 5:436-442, 2016
4.Katoh M. Therapeutics targeting FGF signaling network in human diseases. Trends Pharmacol Sci, 37:1081-1096, 2016
5.Kanemoto K, Fukuta K, Kawai N, Tozawa K, Ochiai M, Okamoto K, Ohnami S, Sakamoto H, Yoshida T, Kanai Y, Katoh M, Yasui T, Kohri K, Kakizoe T, Nakagama H. Genomic Landscape of Experimental Bladder Cancer in Rodents and Its Application to Human Bladder Cancer: Gene Amplification and Potential Overexpression of Cyp2a5/CYP2A6 Are Associated with the Invasive Phenotype. PLoS One, 11:e0167374, 2016
Book
1.Katoh M, Katoh M, Castanet M, Carre A, Polak M. FOXE1: Bamforth-Lazarus syndrome, thyroid dysgenesis and thyroid cancer predisposition. In: Erickson RP, Wynshaw-Boris AJ (eds), Epstein's Inborn Errors of Development: The Molecular Basis of Clinical Disorders and Morphogenesis (3rd ed), USA, Oxford University Press, pp 813-816, 2016