Annual Report 2017
Department of Experimental Therapeutics
Toshihiko Doi, Yasutoshi Kuboki, Kiyotaka Yoh, Yoichi Naito, Takahiro Kogawa, Hideaki Takahashi, Kenichi Harano, Shigehiro Koganemaru, Sumito Shingaki
Introduction
The National Cancer Center - the Exploratory Oncology Research & Clinical Trial Center (NCC-EPOC) Phase I Group has been organized to promote the early drug development especially the first in human (FIH) trial since 2012. The phase I group consists of two sub-units (the NCC Hospital East - Kashiwa & the NCC Hospital -Tsukiji) which are organized by each hospital. The goal of both/each unit is to perform initial clinical evaluation of promising new anti-cancer compounds emerging from laboratories. Our phase I unit is the largest program in Japan, indeed in Asia, and we contribute to the development of new cancer drugs through early phase trials.
In April 2013, the Department of Experimental Therapeutics was launched to strongly promote the EPOC missions as previously described. The members of the Department of Experimental Therapeutics consist of specialists of their oncology fields. Also. we have conducted/contributed to investigator-initiated trial (IIT) using unapproved drugs and academia new seeds.
Our team and what we do
Our team has conducted and managed the early drug development especially the FIH trial.
Research activities
This department plays an important role in new anti-cancer drug development in the NCC as well as in Japan. The top priority is to conduct the FIH trials, while we also perform the phase I trials. Recently, we joined the global phase I trial to accelerate the new drug development in Japan. Web- and tel.-conferences are held with the EU and US sites, and we discuss patient enrollment as well as further developmental strategy. Routine web-conferences are also held between the Kashiwa and Tsukiji Campuses every Friday morning, and we share information about adverse events, patient enrollments, and refer candidates to each other in order to accelerate enrollment.
In the NCC Hospital East, every Thursday, we also share information among all department staff, including Experimental Therapeutics. Several IITs-FIH using new class seeds or unapproved company agents are conducted by each unit.
Clinical trials
In 2017, 40 phase I trials were conducted (Table 1).
Table 1. Phase 1 Trials in 2017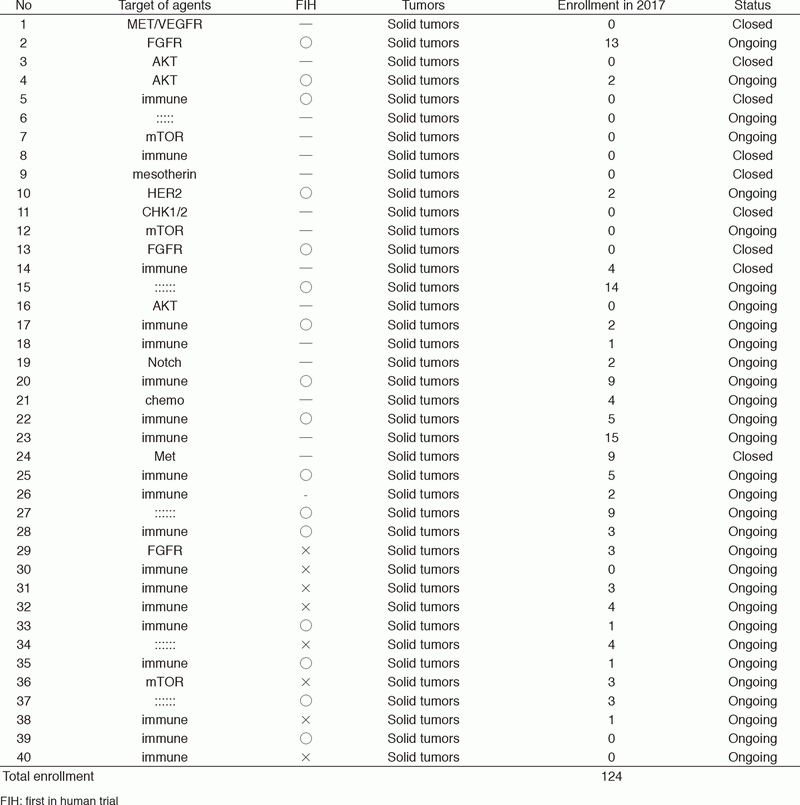
List of papers published in January 2017 - March 2018
Journal
1. Doi T, Hamaguchi T, Shitara K, Iwasa S, Shimada Y, Harada M, Naito K, Hayashi N, Masada A, Ohtsu A. NC-6004 Phase I study in combination with gemcitabine for advanced solid tumors and population PK/PD analysis. Cancer Chemother Pharmacol, 79:569-578, 2017
2. Masuda H, Qi Y, Liu S, Hayashi N, Kogawa T, Hortobagyi GN, Tripathy D, Ueno NT. Reverse phase protein array identification of triple-negative breast cancer subtypes and comparison with mRNA molecular subtypes. Oncotarget, 8:70481-70495, 2017
3. Niho S, Ohe Y, Ohmatsu H, Umemura S, Matsumoto S, Yoh K, Goto K. Switch maintenance chemotherapy using S-1 with or without bevacizumab in patients with advanced non-small cell lung cancer: a phase II study. Lung Cancer, 108:66-71, 2017
4. Miyoshi T, Umemura S, Matsumura Y, Mimaki S, Tada S, Makinoshima H, Ishii G, Udagawa H, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Aokage K, Hishida T, Yoshida J, Nagai K, Goto K, Tsuboi M, Tsuchihara K. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lung. Clinical cancer research, 23:757-765, 2017
5. Sakai D, Chung HC, Oh DY, Park SH, Kadowaki S, Kim YH, Tsuji A, Komatsu Y, Kang YK, Uenaka K, Wijayawardana SR, Wacheck V, Wang X, Yamamura A, Doi T. A non-randomized, open-label, single-arm, Phase 2 study of emibetuzumab in Asian patients with MET diagnostic positive, advanced gastric cancer. Cancer Chemother Pharmacol, 80:1197-1207, 2017
6. Bando H, Rubinstein L, Harris P, Yoshino T, Doi T, Ohtsu A, Welch J, Takebe N. Analysis of esophagogastric cancer patients enrolled in the National Cancer Institute Cancer Therapy Evaluation Program sponsored phase 1 trials. Gastric cancer, 20:481-488, 2017
7. Shitara K, Kim TM, Yokota T, Goto M, Satoh T, Ahn JH, Kim HS, Assadourian S, Gomez C, Harnois M, Hamauchi S, Kudo T, Doi T, Bang YJ. Phase I dose-escalation study of the c-Met tyrosine kinase inhibitor SAR125844 in Asian patients with advanced solid tumors, including patients with MET-amplified gastric cancer. Oncotarget, 8:79546-79555, 2017
8. Hisakane K, Ohmatsu H, Umemura S, Kirita K, Matsumoto S, Yoh K, Niho S, Goto K. Efficacy of Cell-Free and Concentrated Ascites Reinfusion Therapy for Palliative Care in a Patient with Malignant Pleural Mesothelioma: A Case Report. J Nippon Med Sch, 84:231-236, 2017
9. Yoh K, Goto Y, Naito Y, Kishi K, Mori K, Hotta K, Hosomi Y, Yamada K, Tanai C, Tomizawa Y, Inoue A, Hasegawa Y, Nishio M, Ohashi Y, Kunitoh H. Impact of Maintenance Therapy for Patients with Non-small Cell Lung Cancer in a Real-world Setting. Anticancer Res, 37:1507-1513, 2017
10. Ikemura S, Aramaki N, Fujii S, Kirita K, Umemura S, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Kuwata T, Kojima M, Ochiai A, Betsuyaku T, Tsuboi M, Goto K, Ishii G. Changes in the tumor microenvironment during lymphatic metastasis of lung squamous cell carcinoma. Cancer Sci, 108:136-142, 2017
11. Imaoka H, Sasaki M, Takahashi H, Hashimoto Y, Ohno I, Mitsunaga S, Watanabe K, Umemoto K, Kimura G, Suzuki Y, Ikeda M. Progression-free survival as a surrogate endpoint in advanced neuroendocrine neoplasms. Endocr Relat Cancer, 24:475-483, 2017
12. Okuyama H, Ikeda M, Takahashi H, Ohno I, Hashimoto Y, Mitsunaga S, Sakamoto Y, Kondo S, Morizane C, Ueno H, Kobayashi T, Arai Y, Okusaka T. Transarterial (Chemo)Embolization for Liver Metastases in Patients with Neuroendocrine Tumors. Oncology, 92:353-359, 2017
13. Kojima T, Doi T. Immunotherapy for Esophageal Squamous Cell Carcinoma. Curr Oncol Rep, 19:33, 2017
14. Hatogai K, Fujii S, Kojima T, Daiko H, Nomura S, Doi T, Kitano S, Ohtsu A, Takiguchi Y, Yoshino T, Ochiai A. Large-scale comprehensive immunohistochemical biomarker analyses in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol, 143:2351-2361, 2017
15. Doi T, Fuse N, Yoshino T, Kojima T, Bando H, Miyamoto H, Kaneko M, Osada M, Ohtsu A. A Phase I study of intravenous PI3K inhibitor copanlisib in Japanese patients with advanced or refractory solid tumors. Cancer Chemother Pharmacol, 79:89-98, 2017
16. Kuboki Y, Nishina T, Shinozaki E, Yamazaki K, Shitara K, Okamoto W, Kajiwara T, Matsumoto T, Tsushima T, Mochizuki N, Nomura S, Doi T, Sato A, Ohtsu A, Yoshino T. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol, 18:1172-1181, 2017
17. Hisakane K, Yoh K, Nakamura N, Udagawa H, Kirita K, Umemura S, Matsumoto S, Niho S, Akimoto T, Tsuboi M, Goto K. Salvage chemoradiotherapy with cisplatin and vinorelbine for postoperative locoregional recurrence of non-small cell lung cancer. Medicine (Baltimore), 96:e8635, 2017
18. Harano K, Kogawa T, Wu J, Yuan Y, Cohen EN, Lim B, Reuben JM, Ueno NT. Thrombocytosis as a prognostic factor in inflammatory breast cancer. Breast Cancer Res Treat, 166:819-832, 2017
19. Mukai H, Kogawa T, Matsubara N, Naito Y, Sasaki M, Hosono A. A first-in-human Phase 1 study of epirubicin-conjugated polymer micelles (K-912/NC-6300) in patients with advanced or recurrent solid tumors. Invest New Drugs, 35:307-314, 2017
20. Ishibashi M, Neri S, Hashimoto H, Miyashita T, Yoshida T, Nakamura Y, Udagawa H, Kirita K, Matsumoto S, Umemura S, Yoh K, Niho S, Tsuboi M, Masutomi K, Goto K, Ochiai A, Ishii G. CD200-positive cancer associated fibroblasts augment the sensitivity of Epidermal Growth Factor Receptor mutation-positive lung adenocarcinomas to EGFR Tyrosine kinase inhibitors. Sci Rep, 7:46662, 2017
21. Hatogai K, Fujii S, Kojima T, Daiko H, Doi T, Ohtsu A, Ochiai A, Takiguchi Y, Yoshino T. Concordance between PIK3CA mutations in endoscopic biopsy and surgically resected specimens of esophageal squamous cell carcinoma. BMC Cancer, 17:36, 2017
22. Ikeda M, Takahashi H, Kondo S, Lahn MMF, Ogasawara K, Benhadji KA, Fujii H, Ueno H. Phase 1b study of galunisertib in combination with gemcitabine in Japanese patients with metastatic or locally advanced pancreatic cancer. Cancer Chemother Pharmacol, 79:1169-1177, 2017
23. Fujii T, Kogawa T, Wu J, Sahin AA, Liu DD, Chavez-MacGregor M, Giordano SH, Raghavendra A, Murthy RK, Tripathy D, Shen Y, Yamal JM, Ueno NT. Nomogram to predict pathologic complete response in HER2-positive breast cancer treated with neoadjuvant systemic therapy. Br J Cancer, 116:509-514, 2017
24. Torres-Adorno AM, Lee J, Kogawa T, Ordentlich P, Tripathy D, Lim B, Ueno NT. Histone Deacetylase Inhibitor Enhances the Efficacy of MEK Inhibitor through NOXA-Mediated MCL1 Degradation in Triple-Negative and Inflammatory Breast Cancer. Clinical cancer research, 23:4780-4792, 2017
25. Usui Y, Udagawa H, Matsumoto S, Imai K, Ohashi K, Ishibashi M, Kirita K, Umemura S, Yoh K, Niho S, Osame K, Goto K. Association of Serum Anti-GAD Antibody and HLA Haplotypes with Type 1 Diabetes Mellitus Triggered by Nivolumab in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol, 12:e41-e43, 2017
26. Shitara K, Doi T, Nagano O, Fukutani M, Hasegawa H, Nomura S, Sato A, Kuwata T, Asai K, Einaga Y, Tsuchihashi K, Suina K, Maeda Y, Saya H, Ohtsu A. Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407). Gastric cancer, 20:1004-1009, 2017
27. Shitara K, Doi T, Nagano O, Imamura CK, Ozeki T, Ishii Y, Tsuchihashi K, Takahashi S, Nakajima TE, Hironaka S, Fukutani M, Hasegawa H, Nomura S, Sato A, Einaga Y, Kuwata T, Saya H, Ohtsu A. Dose-escalation study for the targeting of CD44v+ cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer, 20:341-349, 2017
28. Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer, 20:407-415, 2017
29. Uemura H, Matsubara N, Kinuya S, Hosono M, Yajima Y, Doi T. Safety and efficacy of radium-223 dichloride in Japanese patients with castration-resistant prostate cancer and bone metastases. Int J Clin Oncol, 22:954-963, 2017
30. Kubota K, Yoshioka H, Oshita F, Hida T, Yoh K, Hayashi H, Kato T, Kaneda H, Yamada K, Tanaka H, Ichinose Y, Park K, Cho EK, Lee KH, Lin CB, Yang JC, Hara K, Asato T, Nakagawa K. Phase III, Randomized, Placebo-Controlled, Double-Blind Trial of Motesanib (AMG-706) in Combination With Paclitaxel and Carboplatin in East Asian Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol, 35:3662-3670, 2017
31. Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med, 5:42-50, 2017
32. Fukuoka S, Kojima T, Koga Y, Yamauchi M, Komatsu M, Komatsuzaki R, Sasaki H, Yasunaga M, Matsumura Y, Doi T, Ohtsu A. Preclinical efficacy of Sym004, novel anti-EGFR antibody mixture, in esophageal squamous cell carcinoma cell lines. Oncotarget, 8:11020-11029, 2017
33. Doi T, Yamaguchi K, Komatsu Y, Muro K, Nishina T, Nakajima TE, Tang R, Yang H, Zhang Y, Jung AS, Ang A, Yasui H. A Phase 1/1b tolerability study of rilotumumab alone or in combination with cisplatin and capecitabine in Japanese patients with gastric cancer. Jpn J Clin Oncol, 47:1002-1009, 2017
34. Wang X, Reyes ME, Zhang D, Funakoshi Y, Trape AP, Gong Y, Kogawa T, Eckhardt BL, Masuda H, Pirman DA Jr, Yang P, Reuben JM, Woodward WA, Bartholomeusz C, Hortobagyi GN, Tripathy D, Ueno NT. EGFR signaling promotes inflammation and cancer stem-like activity in inflammatory breast cancer. Oncotarget, 8:67904-67917, 2017
35. Fukuoka S, Shitara K, Noguchi M, Kawazoe A, Kuboki Y, Bando H, Okamoto W, Kojima T, Doi T, Ohtsu A, Yoshino T. Prophylactic Use of Oral Dexamethasone to Alleviate Fatigue During Regorafenib Treatment for Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer, 16:e39-e44, 2017
36. Yasui H, Go N, Yang H, Amore BM, Jung AS, Doi T. A Phase 1 study evaluating AMG 337 in Asian patients with advanced solid tumors. Jpn J Clin Oncol, 47:772-776, 2017
37. Goto Y, Tanai C, Yoh K, Hosomi Y, Sakai H, Kato T, Kaburagi T, Nishio M, Kim YH, Inoue A, Hasegawa Y, Isobe H, Tomizawa Y, Mori Y, Minato K, Yamada K, Ohashi Y, Kunitoh H. Continuing EGFR-TKI beyond radiological progression in patients with advanced or recurrent, EGFR mutation-positive non-small-cell lung cancer: an observational study. ESMO open, 2:e000214, 2017
38. Udagawa H, Niho S, Kirita K, Umemura S, Matsumoto S, Yoh K, Goto K. Impact of denosumab use on the survival of untreated non-squamous non-small cell lung cancer patients with bone metastases. J Cancer Res Clin Oncol, 143:1075-1082, 2017
39. Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, Kim SW, Kim JH, Planchard D, Felip E, Blackhall F, Haggstrom D, Yoh K, Novello S, Gold K, Hirashima T, Lin CC, Mann H, Cantarini M, Ghiorghiu S, Janne PA. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol, 35:1288-1296, 2017
40. Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N, Kitano A, Jikoh T, Lee C, Fujisaki Y, Ogitani Y, Yver A, Tamura K. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol, 18:1512-1522, 2017
41. Nakaoku T, Kohno T, Araki M, Niho S, Chauhan R, Knowles PP, Tsuchihara K, Matsumoto S, Shimada Y, Mimaki S, Ishii G, Ichikawa H, Nagatoishi S, Tsumoto K, Okuno Y, Yoh K, McDonald NQ, Goto K. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat Commun, 9:625, 2018
42. Shimokawa M, Kogawa T, Shimada T, Saito T, Kumagai H, Ohki M, Kaku T. Overall survival and post-progression survival are potent endpoint in phase III trials of second/third-line chemotherapy for advanced or recurrent epithelial ovarian cancer. J Cancer, 9:872-879, 2018
43. Kuboki Y, Schatz CA, Koechert K, Schubert S, Feng J, Wittemer-Rump S, Ziegelbauer K, Krahn T, Nagatsuma AK, Ochiai A. In situ analysis of FGFR2 mRNA and comparison with FGFR2 gene copy number by dual-color in situ hybridization in a large cohort of gastric cancer patients. Gastric cancer, 21:401-412, 2018
44. Doi T, Hewes B, Kakizume T, Tajima T, Ishikawa N, Yamada Y. Phase I study of single-agent ribociclib in Japanese patients with advanced solid tumors. Cancer Sci, 109:193-198, 2018
45. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, Bennouna J. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol, 36:61-67, 2018
46. Seto T, Hirai F, Saka H, Kogure Y, Yoh K, Niho S, Fukase K, Shimada H, Sasai M, Fukino K. Safety and tolerability of selumetinib as a monotherapy, or in combination with docetaxel as second-line therapy, in Japanese patients with advanced solid malignancies or non-small cell lung cancer. Jpn J Clin Oncol, 48:31-42, 2018
47. Fujii T, Matsuda N, Kono M, Harano K, Chen H, Luthra R, Roy-Chowdhuri S, Sahin AA, Wathoo C, Joon AY, Tripathy D, Meric-Bernstam F, Ueno NT. Prior systemic treatment increased the incidence of somatic mutations in metastatic breast cancer. Eur J Cancer, 89:64-71, 2018
48. Matsumoto H, Kawazoe A, Shimada K, Fukuoka S, Kuboki Y, Bando H, Kojima T, Ohtsu A, Yoshino T, Doi T, Shitara K. A retrospective study of the safety and efficacy of paclitaxel plus ramucirumab in patients with advanced or recurrent gastric cancer with ascites. BMC Cancer, 18:120, 2018
49. Taira T, Yoh K, Nagase S, Kubota K, Ohmatsu H, Niho S, Onozawa M, Akimoto T, Ohe Y, Goto K. Long-term results of S-1 plus cisplatin with concurrent thoracic radiotherapy for locally advanced non-small-cell lung cancer. Cancer Chemother Pharmacol, 81:565-572, 2018
