Annual Report 2017
Department of Gastric Surgery
Hitoshi Katai, Takeo Fukagawa, Shinji Morita, Sho Otsuki, Yukinori Yamagata, Masahiro Yura, Yuya Sato, Maiko Ito, Toshirou Nishida
Introduction
The Department of Gastric Surgery treats not only gastric adenocarcinoma but also sarcomas of gastric origin, such as malignant lymphomas or gastrointestinal stromal tumors (GISTs). Principally, we treat tumors of the esophagogastric junction.
Our team and what we do
Our department includes six staff surgeons, two chief residents and three or four rotating residents at any given time. Nine to eleven patients are operated upon every week. Our department shares a ward with the Department of Hepatobiliary and Pancreatic Surgery and the Department of Gastrointestinal Medical Oncology. Patients with stage I disease are followed-up without adjuvant chemotherapy. Adjuvant S-1 chemotherapy is used for patients with stage II and III disease. Adjuvant XELOX chemotherapy is applied for stage IIIC disease. Neoadjuvant chemotherapy is frequently used for patients with locally advanced tumors.
Approximately 50% of patients with a superficial adenocarcinoma lesion are treated with endoscopic submucosal dissection (ESD). Some undergo subsequent surgery based on the histological findings of the resected specimen. Every Tuesday from 6:15 to 7:00 P.M., a clinical conference is held for surgeons, a medical oncologist, and endoscopists. All patients with gastric malignancies in the ward or on the waiting list for admission are briefly reviewed and those whose treatment is controversial are discussed in detail. Every Friday between 7:15 and 8:30 A.M., another clinical conference is held, in which surgeons and endoscopists present all candidates for surgical and endoscopic treatment for the following week, and the treatment strategy for each case is discussed in detail. On the third Wednesday of every month, between 6:00 and 7:00 P.M., pathological conference is held, in which surgeons, endoscopists and pathologists discuss. These conferences are held in English whenever a foreign guest doctor is present.
We consider the education of foreign surgeons to be an important function. In 2017, more than 20 surgeons from various countries visited this department for one week to eight months to learn about the management of gastric cancer patients, especially surgical techniques for lymph node dissection and postoperative care. All staff surgeons have sufficient experience in teaching in English.
Research activities
Several translational studies are being carried out in cooperation with the National Cancer Center Research Institute (NCCRI). Genomic scanning in gastric cancer is being carried out. DNA methylation as a gastric cancer metastasis risk factor has been investigated. A mini-chip assay of peritoneal washings for prediction of gastric cancer recurrence is being developed. Research on the detection of small amounts of cancer cells in peripheral blood and bone marrow of gastric cancer patients is being carried out. The study of the correlation of micro-RNA profiles and prognosis has started.
Clinical trial
Our department has been playing a central role in conducting multi-institutional clinical trials. H. Katai is a representative of the Gastric Cancer Surgical Study Group of the Japan Clinical Oncology Group (JCOG). Patients with gastric cancer are, when eligible, invited to participate in one of the ongoing clinical trials mentioned below. The JCOG 0501 phase III trial to evaluate the effect of neo-adjuvant (S-1 and CDDP) and adjuvant chemotherapy (S-1) for large type III and type IV tumors was completed and the results were reported. The JCOG 1001, which is designed to evaluate the significance of bursectomy for advanced cancer, was carried out and the results were reported. The JCOG 0912 phase III trial to prove the non-inferiority of laparoscopic gastrectomy over its open counterpart for patients with clinical stage IA and IB gastric cancer has been completed for accrual. The phase II trial to prove the feasibility of laparoscopic total and proximal gastrectomy for stage IA and IB gastric cancer (JCOG 1401) has also been completed for accrual and the results were reported.
The results of JCOG 1104 phase III trial to evaluate the optimal period of adjuvant S-1 chemotherapy for pathological stage II gastric cancer patients who underwent D2 gastrectomy were reported. The JCOG1301C, a randomized phase II study of systemic chemotherapy with and without trastuzumab followed by surgery in HER2 positive advanced gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis is ongoing.
The JCOG 1507 phase III trial to confirm S-1 adjuvant chemotherapy for pathological stage II/III vulnerable elderly gastric cancer patients, and the JCOG 1509 phase III trial to evaluate the efficacy of neoadjuvant chemotherapy with S-1 plus oxalliplatin followed by D2 gastrectomy with adjuvant S-1 in locally advanced gastric cancer are ongoing.
Education
Education of surgical operations has been introduced for chief and rotating residents throughout the perioperative management of more than 500 gastric cancer patients.
Future prospects
D2 gastrectomy is considered the standard surgical treatment for advanced gastric cancer but multi-modality treatments combined with surgery will further improve survival rates. The efficacy of paraaortic node dissection is still controversial. There are several surgical options for early gastric cancer depending on the risk of nodal metastasis. The efficacy of laparoscopic surgery for early gastric cancer is being assessed. Moreover, robotic surgery is introduced as advanced medical care services and the safety and effectiveness have currently been evaluated. These procedures will require good quality control achieved through supervision and training by experienced surgeons in high volume centers.
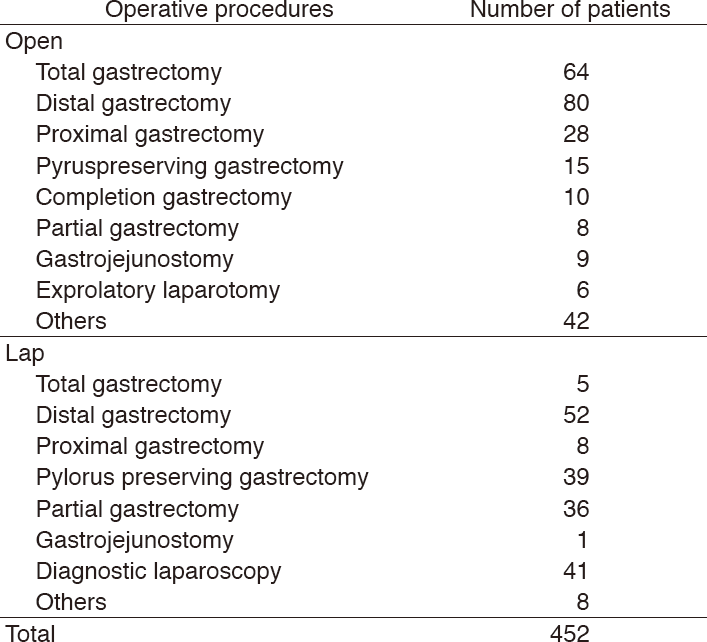
Table 1. Number of patients treated in 2017

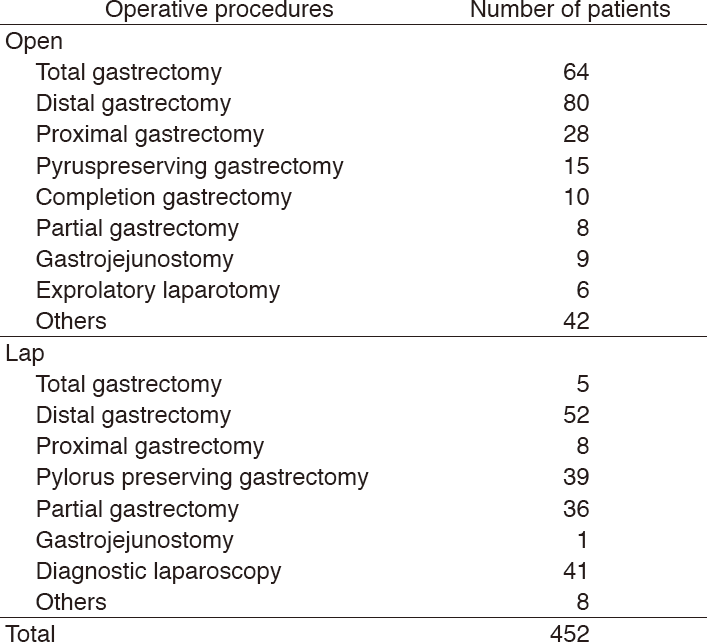
Table 3. Morbidity and mortality after gastrectomy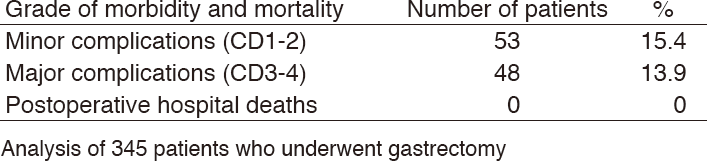
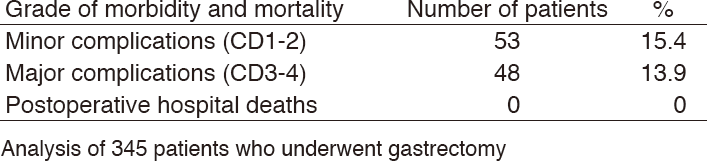
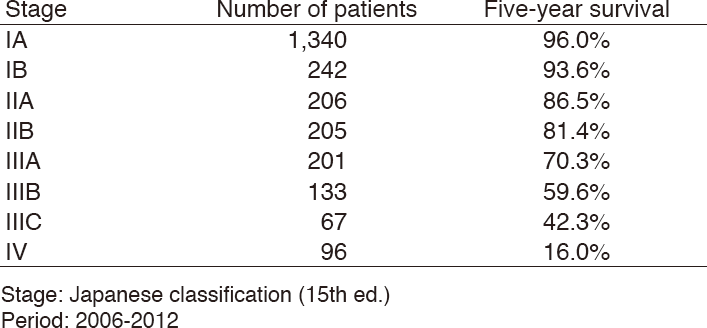
Table 4. Five-year overall survival rate for each stage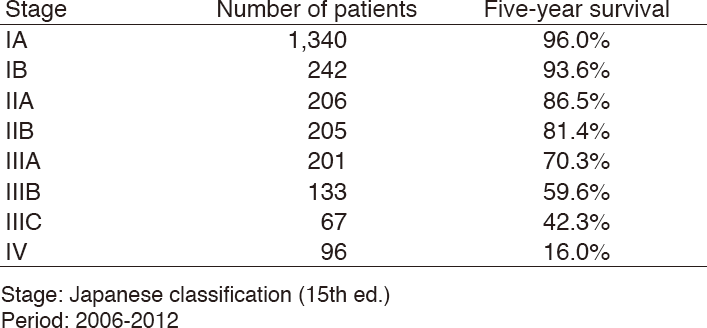
List of papers published in January 2017 - March 2018
Journal
1. Ichikawa W, Terashima M, Ochiai A, Kitada K, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M. Impact of insulin-like growth factor-1 receptor and amphiregulin expression on survival in patients with stage II/III gastric cancer enrolled in the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer. Gastric cancer, 20:263-273, 2017
2. Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg, 265:277-283, 2017
3. Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A, Nakamori M, Onaya H, Sasako M. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric cancer, 20:322-331, 2017
4. Niinuma T, Kai M, Kitajima H, Yamamoto E, Harada T, Maruyama R, Nobuoka T, Nishida T, Kanda T, Hasegawa T, Tokino T, Sugai T, Shinomura Y, Nakase H, Suzuki H. Downregulation of miR-186 is associated with metastatic recurrence of gastrointestinal stromal tumors. Oncology letters, 14:5703-5710, 2017
5. Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric cancer, 20:699-708, 2017
6. Sugawara S, Arai Y, Sone M, Katai H. Frequency, Severity, and Risk Factors for Acute Pancreatitis After Percutaneous Transhepatic Biliary Stent Placement Across the Papilla of Vater. Cardiovasc Intervent Radiol, 40:1904-1910, 2017
7. Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, Hirao M, Yoshida K, Oki E, Sasako M, Emi Y, Tsujinaka T. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer, 20:175-181, 2017
8. Nishida T. Therapeutic strategies for wild-type gastrointestinal stromal tumor: is it different from KIT or PDGFRA-mutated GISTs? Translational gastroenterology and hepatology, 2:92, 2017
9. Takahashi T, Elzawahry A, Mimaki S, Furukawa E, Nakatsuka R, Nakamura H, Nishigaki T, Serada S, Naka T, Hirota S, Shibata T, Tsuchihara K, Nishida T, Kato M. Genomic and transcriptomic analysis of imatinib resistance in gastrointestinal stromal tumors. Genes Chromosomes Cancer, 56:303-313, 2017
10. Ohara K, Arai E, Takahashi Y, Ito N, Shibuya A, Tsuta K, Kushima R, Tsuda H, Ojima H, Fujimoto H, Watanabe SI, Katai H, Kinoshita T, Shibata T, Kohno T, Kanai Y. Genes involved in development and differentiation are commonly methylated in cancers derived from multiple organs: a single-institutional methylome analysis using 1007 tissue specimens. Carcinogenesis, 38:241-251, 2017
11. Suzuki H, Oda I, Abe S, Sekiguchi M, Nonaka S, Yoshinaga S, Saito Y, Fukagawa T, Katai H. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer, 20:679-689, 2017
12. Kanda T, Masuzawa T, Hirai T, Ikawa O, Takagane A, Hata Y, Ojima H, Sodeyama H, Mochizuki I, Ishikawa T, Kagimura T, Nishida T. Surgery and imatinib therapy for liver oligometastasis of GIST: a study of Japanese Study Group on GIST. Jpn J Clin Oncol, 47:369-372, 2017
13. Wada T, Fujiwara H, Morita S, Fukagawa T, Katai H. Incidence of and risk factors for preoperative deep venous thrombosis in patients undergoing gastric cancer surgery. Gastric cancer, 20:872-877, 2017
14. Terashima M, Ichikawa W, Ochiai A, Kitada K, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M. TOP2A, GGH, and PECAM1 are associated with hematogenous, lymph node, and peritoneal recurrence in stage II/III gastric cancer patients enrolled in the ACTS-GC study. Oncotarget, 8:57574-57582, 2017
15. Wada N, Takahashi T, Kurokawa Y, Nakajima K, Masuzawa T, Nakatsuka R, Kawada J, Nishida T, Kimura Y, Tanaka K, Miyazaki Y, Makino T, Yamasaki M, Takiguchi S, Mori M, Doki Y. Appropriate Follow-Up Strategies for Gastrointestinal Stromal Tumor Patients Based on the Analysis of Recurrent Interval and Patterns. Digestion, 95:115-121, 2017
16. Kurokawa Y, Yang HK, Cho H, Ryu MH, Masuzawa T, Park SR, Matsumoto S, Lee HJ, Honda H, Kwon OK, Ishikawa T, Lee KH, Nabeshima K, Kong SH, Shimokawa T, Yook JH, Doki Y, Im SA, Hirota S, Hahn S, Nishida T, Kang YK. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer, 117:25-32, 2017
17. Kaito A, Kinoshita T, Shitara K, Shibasaki H, Nishida T. Timing of initiation of adjuvant chemotherapy for gastric cancer: A case-matched comparison study of laparoscopic vs. open surgery. Eur J Surg Oncol, 43:801-807, 2017
18. Obata Y, Horikawa K, Takahashi T, Akieda Y, Tsujimoto M, Fletcher JA, Esumi H, Nishida T, Abe R. Oncogenic signaling by Kit tyrosine kinase occurs selectively on the Golgi apparatus in gastrointestinal stromal tumors. Oncogene, 36:3661-3672, 2017
19. Miyakita Y, Ohno M, Takahashi M, Muragaki Y, Katai H, Narita Y. Immunochemotherapy using rituximab (RTX) and high-dose methotrexate (HD-MTX): an evaluation of the addition of RTX to HD-MTX in recurrent primary central nervous system lymphoma (PCNSL). Jpn J Clin Oncol, 47:919-924, 2017
20. Tanabe S, Hirabayashi S, Oda I, Ono H, Nashimoto A, Isobe Y, Miyashiro I, Tsujitani S, Seto Y, Fukagawa T, Nunobe S, Furukawa H, Kodera Y, Kaminishi M, Katai H. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric cancer, 20:834-842, 2017
21. Nishida T. Asian consensus guidelines for gastrointestinal stromal tumor: what is the same and what is different from global guidelines. Translational gastroenterology and hepatology, 3:11, 2018