Annual Report 2017
Department of Gastrointestinal Medical Oncology
Narikazu Boku, Ken Kato, Atsuo Takashima, Yoshitaka Honma, Satoru Iwasa, Hirokazu Shoji, Hidekazu Hirano, Natsuko Okita, Takahiro Miyamoto.
Introduction
The Department of Gastrointestinal Medical Oncology focuses on the development of new drugs and the establishment of standard chemotherapy regimens including multi-modality treatment with surgery and/or radiotherapy for advanced Esophageal/Gastric/Colorectal/Head & Neck cancers, gastrointestinal stromal tumor (GIST), and other gastrointestinal (GI) malignancies.
Over recent years, molecular-target agents have been developed for GI cancer. Anti-angiogenetic agents, bevacizumab (BV) and aflibercept which direct against vascular endothelial growth factor (VEGF) have been used for colorectal cancer, and anti-VEGF receptor-2 (VEGFR-2) antibody, ramcirumab (RAM) for gastric and colorectal cancers. Meanwhile, anti-epidermal growth factor receptor (EGFR) anti-bodies, such as cetuximab and panitumumab, have been used for RAS-wild type colorectal cancer (cetuximab for Head & Neck cancer). Moreover, trastuzumab has been used for HER2-positive gastric cancer, and multi-kinase inhibitors, such as imatinib, sunitinib, and regorafenib, have also been used for GIST and colorectal cancer. As the main topic in recent years, the efficacy of the im-mune checkpoint inhibitor has also been evaluated for GI and Head & Neck cancers, and Nivolumab, anti-programed cell death 1 (PD-1) antibody, was approved for gastric cancer and Head & Neck cancer as salvage-line treatment in 2017.
We expect to develop other novel agents for the treatment of metastatic GI and Head & Neck cancers that inhibit intracellular signal transduction, cellular interactions, and immunogenic interactions in the near future. However, many unusual adverse effects and a marked increase in medical costs have led to extensive discussion on biomarkers for more accurate targeting of the optimal target population. Although the response rates of monotherapy with these molecular-targeted drugs up to now have not been so high (about 5% to 20%) when used broadly in non-selected patients, there are a few new candidate biomarkers that may be useful for identifying patients for whom these molecular-targeted drugs will be beneficial. For example, RAS mutation is one of negative predictive markers for response to anti-EGFR antibody, and it is expected that the expression of programed cell death-ligand 1 (PD-L1) in cancer cells or antigen-presenting cells may be one of positive predictive markers for response to anti-PD-1 antibody. Accordingly, the identification of molecular markers that can be used to predict tumor shrinkage and/or prolong prognosis will be critical for the further progress in treatment of GI and Head & Neck malignancies.
Routine activities
The staff of the Department of GI Medical Oncology consists of eight medical oncologists, one chief resident, and three or four residents. We hold a daily case conference together at 5 p.m. after finishing routine clinical work, and also hold a weekly research conference for sharing and discussing the progress of clinical studies and in-house researches. Multidisciplinary meetings with surgery departments (Colorectal, Gastric, Esophageal), and the Departments of Head and Neck Oncology, and Radiation Oncology are held weekly to decide optimal treatment strategies for each patient and to discuss treatment consensus for the specific disease. Palliative care considering the physical and psychological aspects of each case is another important issue discussed in these meetings with other medical staff. The palliative care team and psycho-oncologists advise us on how to minimize patients' discomfort and anxiety throughout end-of-life care. In 2017, we treated 2,226 hospitalized patients (935 of whom were newly diagnosed). Of these patients, 147 were enrolled into protocol studies including two investigator-initiated trials according to the Good Clinical Practice (GCP).
Research activities
An endoscopic biopsy and blood sampling before and after chemotherapy provide an excellent opportunity to study biomarkers related to therapy-induced tumor response, overall survival, and time to progression or recurrence. We are collecting these fresh blood and tissue samples from patients with GI and Head & Neck cancers to explore biomarkers, which are related to patients' outcome, such as gene expression, micro RNA or immunogenic profiles in collaboration with basic researchers using genome sequencing, immune-panel, microarray or real time PCR techniques.
We also measure the immunohistochemical expressions of possible predictive biomarkers using paraffin-embedded GI and Head & Neck cancer specimens obtained from surgical resection or endoscopic biopsy and investigated the correlation to anti-cancer drug metabolism and clinical outcomes. These studies are being performed in collaboration with the National Cancer Center Research Institute, or other institutions.
Clinical trials
We are conducting several clinical trials in collaboration with the surgery departments and the Department of Radiation Oncology in our hospital and other institutes, including the Japan Clinical Oncology Group (JCOG) trials, the West Japan Oncology Group (WJOG) trials, company-initiated trials, and other collaborative investigator-initiated trials. The details of clinical trials are summarized in Table 1.
1. Colorectal and Anal Canal Cancer
In the first-line treatment, the result of a multicenter phase III trial (TRICOLORE), investigating whether S-1+irinotecan+BV (SIRB) regimen is non-inferior to capecitabine + oxaliplatin (XELOX) plus BV has been published and the non-inferiority of SIRB regimen was demonstrated. A phase III trial (PARADIGM) comparing 5-fluorouracil + l-leucovorin + oxaliplatin (FOLFOX) + panitumumab with FOLFOX+BV in RAS wild type population, and another clinical trial comparing pembrolizumab, an anti-PD-1 antibody, with standard chemotherapy for untreated MSI-high colorectal cancer, finished patient accrual in 2017. A new randomized phase II trial (WJOG9216G) comparing FOLFOX + irinotecan (FOLFOXIRI) + RAM with 5-fluorouracil + l-leucovorin + irinotecan (FOLFIRI) + RAM has been started in 2017. A new phase II trial (Hybrid) for RAS wild type population which investigates the efficacy and feasibility of the new treatment strategy switching a molecular-target agent from cetuximab to BV according to the initial response to FOLFIRI + cetuximab and a phase III trial (JCOG1018) to investigate the superiority of fluoropyrimidine + oxaliplatin + BV to fluoropyrimidine + BV targeted at frail or elderly patients are ongoing.
In the second-line treatment, a phase III trial (AXEPT) investigating the non-inferiority of XELIRI (capecitabine + irinotecan) to FOLFIRI for patients with failure of first-line treatment with FOLFOX or XELOX + BV showed the non-inferiority of XELIRI regimen. A phase III trial to evaluate the additive effect of BBI608 (an inhibitor targeting at cancer stem cell) combined with FOLFIRI finished patient accrual in 2017.
In the salvage-line treatment, an investigator-initiated phase II trial (Lemon) to evaluate the efficacy and feasibility of lenvatinib for patients after failure of standard chemotherapy except regorafenib finished patient accrual in 2017. Another investigator-initiated phase II trial to evaluate the efficacy and feasibility of trastuzumab + pertuzumab for HER2-positive colorectal cancer will start in early 2018. A phase II trial (WJOG8916G) investigating efficacy and feasibility of FTD/TPI + cetuximab treatment for cetuximab resistant population is ongoing.
As an adjuvant treatment, a phase III trial (JCOG0603) comparing adjuvant FOLFOX with observation after complete resection of liver metastasis from colorectal cancer is underway. A randomized controlled trial (JCOG1310) comparing between postoperative and perioperative chemotherapy with FOLFOX chemotherapy for lateral lymph node positive lower rectal cancer is also under registration.
The phase II part of a phase I/II trial (JCOG0903) of definitive chemoradiotherapy with S-1 + mitomycin C for locally advanced anal canal squamous cell carcinoma finished patient accrual.
2. Gastric cancer
In neoadjuvant/adjuvant settings, an investigator-initiated phase I/II trial (APOLLO-11) of perioperative TAS-118 (S-1 plus l-leucovorin) ± oxaliplatin, and a placebo controlled company-initiated phase III trial (ATTRACTION-4) to evaluate the additive effect of nivolumab, anti-PD-1 antibody, combined with adjuvant S-1 or XELOX, and a phase III trial (JCOG1509) investigating the additive effect of neoadjuvant S-1 plus oxaliplatin (SOX) for stage III gastric cancer are ongoing in cooperation with the Department of Gastric Surgery.
In the first-line treatment, the final results of a phase III trial (JCOG1013) comparing S-1/CDDP/Docetaxel (DCS) with S-1/CDDP (CS) will be published in ASCO 2018 meeting. Other ongoing trials which finished patient accrual in 2017 are as follows: 1) SOLAR trial comparing TAS-118 plus oxaliplatin comparing with CS; 2) JCOG1108 trial comparing FLTAX (5-fluorouracil + l-leucovorin + taxol) with FL (5-fluorouracil + l-leucovorin) for patients who are unfit for S-1 and/or CDDP usage due to severe peritoneal dissemination; 3) Phase II part of ATTRACTION-5 trial (phase II/III trial) investigating the feasibility of nivolumab with SOX or XELOX; 4) Check Mate-44 trial comparing nivolumab plus ipilimumab (anti-CTLA-4 antibody) with fluoropyrimidine plus oxaliplatin; and 5) Javelin Gastric 100 trial comparing XELOX continuation with avelumab (anti-PD-L1 antibody) maintenance treatment after disease stabilization using XELOX. For elderly population, a randomized phase II trial (WJOG8315G), comparing S-1 monotherapy with SOX is ongoing.
In the second-line treatment, a new investigator-initiated phase II trial to evaluate the efficacy and feasibility of nivolumab plus RAM finished patient accrual on schedule in 2017.
In the salvage-line treatment, a phase III trial (Javelin Gastric 300) comparing avelumab with BSC ± physician choice chemotherapy failed to show superiority of avelumab to standard treatment. A new phase III trial (RINDBERG) investigating the additive effect of RAM to CPT-11 for fluoropyrimidine, platinum, taxane, and RAM resistant gastric cancer patients has started. A new global phase III trial (ANGEL) comparing apatinib, a tyrosine kinase inhibitor that selectively inhibits the VEGFR-2, with placebo for heavily treated gastric cancer also started in 2017. Multi-institutional collaborative translational research (WJOG10417GTR) exploring biomarkers of nivolumb in the salvage-line treatment for advanced gastric cancer using fresh biopsy and blood samples in collaboration with the Department of Immune Medicine in the National Cancer Center Research Institute (NCCRI) will start in 2018.
For HER2 positive gastric cancer, a phase II trial (JCOG1301C) of neoadjuvant CS + trastuzumab for locally advanced disease is ongoing under Advanced Medical Care B program. In metastatic setting, the result of phase III trial (JACOB) which evaluates the additive effect of pertuzumab combined with capecitabine and cisplatin plus trastuzumab in the first-line treatment was published in the ESMO 2017 congress and failed to show superiority of pertuzumab combination treatment. A multi-center feasibility study of S-1/oxaliplatin plus trastuzumab in the first-line treatment finished patient accrual in 2017. In the second-line treatment, a randomized phase II trial (WJOG 8315G) to evaluate the additive effect of trastuzumab with paclitaxel beyond progression finished patient accrual and the final results will be published at the annual meeting of ASCO in 2018. A new randomized phase II trial of comparing DS-8201a, antibody-drug conjugate of trastuzumab and deruxtecan, with irinotecan in the third-line setting has started.
3. Esophageal Cancer
In neoadjuvant/adjuvant settings, a phase III trial (JCOG1109) comparing preoperative Docetaxel + 5-FU + CDDP (DCF) or 5-FU + CDDP (CF) plus radiotherapy (CF-RT, 41.4Gy) with standard CF for resectable stage IB/II/III esophageal cancer, and a placebo controlled phase III trial to evaluate the additive effect of nivolumab as adjuvant treatment after curative resection following neoadjuvant chemoradiotherapy are ongoing.
For definitive CRT as a non-surgical treatment, the final results of phase II study (JCOG0909) investigating the efficacy of CF-RT (50.4 Gy) regimen followed by salvage surgery or endoscopic resection for stage IB/II/III esophageal cancer will be published at the annual meeting of ASCO in 2018. A new phase III trial investigating the additive effect of induction chemotherapy with DCF to definitive CF-RT (60Gy) for locally far-advanced esophageal cancer will start in early 2018.
In the first-line treatment, a phase III trial (JCOG1314) comparing biweekly DCF with standard CF regimen is ongoing. Moreover, two randomized controlled trials to investigate the efficacy of immune checkpoint inhibitor have started as follows: 1) CheckMate-468 comparing efficacy among nivolumab + ipilimumab, CF + nivolumab and CF and 2) KEYNOTE-590 investigating additive effect of pembrolizumab to CF.
In the second-line treatment, two randomized controlled trials to investigate the efficacy of immune checkpoint inhibitor finished patient accrual in 2017 as follows: 1) OPERA trial comparing Taxan versus nivolumab and 2) KEYNOTE-181 trial comparing paclitaxel versus pembrolizumab.
In the salvage-line treatment, a feasibility study to investigate the efficacy of pembrolizumab also finished patient accrual in 2017.
4. Head & Neck Cancer
For head and neck squamous cell carcinoma (HNSCC), collaborating with the Department of Head and Neck Oncolory and the Department of Radiation Oncology, a phase III trial investigating the additive effect of avelumab to definitive CDDP + RT for locally advanced HNSCC, and a phase III trial comparing nivolumab plus ipilimumab with standard chemotherapy (CF plus cetuximab) as the first-line treatment for metastatic HNSCC, are ongoing. A phase III trial comparing MEDI-4736 (anti-PD-L1 antibody) / Tremelimumab (anti-CTLA-4 antibody), MEDI-4736, and standard chemotherapy (Docetaxel or Cetuximab or S-1) in the second-line setting finished patient accrual in 2017.
5. Others
For metastatic neuroendocrine carcinoma (NEC) in GI-tract or hepato-billiary-Pancreatic field, a phase III trial (JCOG1213) comparing irinotecan plus CDDP with etoposide plus CDDP as the first-line treatment is progressing faster than planned. A new phase III trial (JCOG1502C) for curatively resected small bowel adenocarcinoma, comparing adjuvant XELOX with surgery alone has started under Advanced Medical Care B program in global cooperation with the International Rare Cancers Initiative (IRCI). An investigator-initiated phase II trial of regorafenib for imatinib-resistant GIST finished patient accrual in 2017. Several other clinical trials have also been conducted and eligible patients have been enrolled as shown in Table 1.
Table 1. Clinical trials conducted in 2017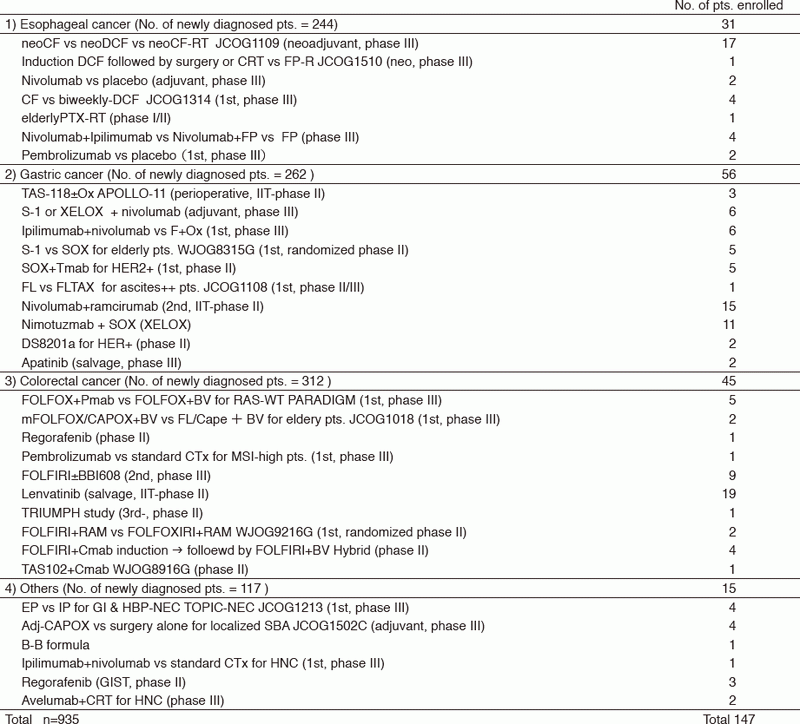
List of papers published in January 2017 - March 2018
Journal
1.Mizukami T, Togashi Y, Naruki S, Banno E, Terashima M, de Velasco MA, Sakai K, Yoneshige A, Hayashi H, Fujita Y, Tomida S, Nakajima TE, Fujino T, Boku N, Ito A, Nakagawa K, Nishio K. Significance of FGF9 gene in resistance to anti-EGFR therapies targeting colorectal cancer: A subset of colorectal cancer patients with FGF9 upregulation may be resistant to anti-EGFR therapies. Mol Carcinog, 56:106-117, 2017
2.Nishikawa K, Takahashi T, Takaishi H, Miki A, Noshiro H, Yoshikawa T, Nishida Y, Iwasa S, Miwa H, Masuishi T, Boku N, Yamada Y, Kodera Y, Yoshida K, Morita S, Sakamoto J, Saji S, Kitagawa Y. Phase II study of the effectiveness and safety of trastuzumab and paclitaxel for taxane- and trastuzumab-naive patients with HER2-positive, previously treated, advanced, or recurrent gastric cancer (JFMC45-1102). Int J Cancer, 140:188-196, 2017
3.Yamamoto N, Fujiwara Y, Tamura K, Kondo S, Iwasa S, Tanabe Y, Horiike A, Yanagitani N, Kitazono S, Inatani M, Tanaka J, Nishio M. Phase Ia/Ib study of the pan-class I PI3K inhibitor pictilisib (GDC-0941) administered as a single agent in Japanese patients with solid tumors and in combination in Japanese patients with non-squamous non-small cell lung cancer. Invest New Drugs, 35:37-46, 2017
4.Ohue M, Iwasa S, Kanemitsu Y, Hamaguchi T, Shiozawa M, Ito M, Yasui M, Katayama H, Mizusawa J, Shimada Y. A Phase II/III randomized controlled trial comparing perioperative versus postoperative chemotherapy with mFOLFOX6 for lower rectal cancer with suspected lateral pelvic node metastasis: Japan Clinical Oncology Group Study JCOG1310 (PRECIOUS study). Jpn J Clin Oncol, 47:84-87, 2017
5.Fukuoka K, Nara S, Honma Y, Kishi Y, Esaki M, Shimada K. Hepatectomy for Colorectal Cancer Liver Metastases in the Era of Modern Preoperative Chemotherapy: Evaluation of Postoperative Complications. World J Surg, 41:1073-1081, 2017
6.Shiono S, Okumura T, Boku N, Hishida T, Ohde Y, Sakao Y, Yoshiya K, Hyodo I, Mori K, Kondo H. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg, 51:504-510, 2017
7.Nishimura T, Iwasa S, Nagashima K, Okita N, Takashima A, Honma Y, Kato K, Hamaguchi T, Yamada Y, Shimada Y, Boku N. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer, 20:655-662, 2017
8.Yoshino T, Hyodo I, Nishina T, Narahara H, Sugimoto N, Yoshisue K, Boku N. Phase I clinical and pharmacokinetic study of S-1 plus oral leucovorin in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol, 79:107-116, 2017
9.Saka H, Kitagawa C, Kogure Y, Takahashi Y, Fujikawa K, Sagawa T, Iwasa S, Takahashi N, Fukao T, Tchinou C, Landers D, Yamada Y. Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: a Phase I study. Invest New Drugs, 35:451-462, 2017
10.Kataoka K, Kinoshita T, Moehler M, Mauer M, Shitara K, Wagner AD, Schrauwen S, Yoshikawa T, Roviello F, Tokunaga M, Boku N, Ducreux M, Terashima M, Lordick F. Current management of liver metastases from gastric cancer: what is common practice? New challenge of EORTC and JCOG. Gastric Cancer, 20:904-912, 2017
11.Nishiumi S, Kobayashi T, Kawana S, Unno Y, Sakai T, Okamoto K, Yamada Y, Sudo K, Yamaji T, Saito Y, Kanemitsu Y, Okita NT, Saito H, Tsugane S, Azuma T, Ojima N, Yoshida M. Investigations in the possibility of early detection of colorectal cancer by gas chromatography/triple-quadrupole mass spectrometry. Oncotarget, 8:17115-17126, 2017
12.Takahari D, Mizusawa J, Koizumi W, Hyodo I, Boku N. Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric Cancer, 20:757-763, 2017
13.Okusaka T, Miyakawa H, Fujii H, Nakamori S, Satoh T, Hamamoto Y, Ito T, Maguchi H, Matsumoto S, Ueno H, Ioka T, Boku N, Egawa S, Hatori T, Furuse J, Mizumoto K, Ohkawa S, Yamaguchi T, Yamao K, Funakoshi A, Chen JS, Cheng AL, Sato A, Ohashi Y, Tanaka M. Updated results from GEST study: a randomized, three-arm phase III study for advanced pancreatic cancer. J Cancer Res Clin Oncol, 143:1053-1059, 2017
14.Doi T, Hamaguchi T, Shitara K, Iwasa S, Shimada Y, Harada M, Naito K, Hayashi N, Masada A, Ohtsu A. NC-6004 Phase I study in combination with gemcitabine for advanced solid tumors and population PK/PD analysis. Cancer Chemother Pharmacol, 79:569-578, 2017
15.Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Doki Y, Kitagawa Y. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol, 18:631-639, 2017
16.Nomura M, Kato K, Ando N, Ohtsu A, Muro K, Igaki H, Abe T, Takeuchi H, Daiko H, Gotoh M, Kataoka K, Wakabayashi M, Kitagawa Y. Comparison between neoadjuvant chemotherapy followed by surgery and definitive chemoradiotherapy for overall survival in patients with clinical Stage II/III esophageal squamous cell carcinoma (JCOG1406-A). Jpn J Clin Oncol, 47:480-486, 2017
17.Takashima A, Iizumi S, Boku N. Survival after failure of first-line chemotherapy in advanced gastric cancer patients: differences between Japan and the rest of the world. Jpn J Clin Oncol, 47:583-589, 2017
18.Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K, Ueda S, Yoshida K, Shimodaira H, Nishina T, Tsuda M, Kurokawa Y, Tamura T, Sasaki Y, Morita S, Koizumi W. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol, 2:277-287, 2017
19.Katakami N, Oda K, Tauchi K, Nakata K, Shinozaki K, Yokota T, Suzuki Y, Narabayashi M, Boku N. Phase IIb, Randomized, Double-Blind, Placebo-Controlled Study of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients With Cancer. J Clin Oncol, 35:1921-1928, 2017
20. Katsuya Y, Honma Y, Taniguchi H, Kato K, Okita N, Takashima A, Iwasa S, Hamaguchi T, Boku N, Umezawa R, Inaba K, Ito Y, Itami J, Koyanagi K, Igaki H, Tachimori Y. Clinicopathological features and pathological evaluation of preoperative treatment of patients with resectable esophageal carcinosarcoma. Esophagus, 14:317-323, 2017
21.Ito T, Honma Y, Hijioka S, Kudo A, Fukutomi A, Nozaki A, Kimura Y, Motoi F, Isayama H, Komoto I, Hisamatsu S, Nakajima A, Shimatsu A. Phase II study of lanreotide autogel in Japanese patients with unresectable or metastatic well-differentiated neuroendocrine tumors. Invest New Drugs, 35:499-508, 2017
22.Okumura T, Boku N, Hishida T, Ohde Y, Sakao Y, Yoshiya K, Higashiyama M, Hyodo I, Mori K, Kondo H. Surgical Outcome and Prognostic Stratification for Pulmonary Metastasis From Colorectal Cancer. Ann Thorac Surg, 104:979-987, 2017
23.Watanabe S, Takashima A, Taniguchi H, Tanaka Y, Nakamura S, Okita N, Honma Y, Iwasa S, Kato K, Hamaguchi T, Boku N. Esophageal Metastasis from Rectal Cancer Successfully Treated with Fluorouracil-Based Chemotherapy with Bevacizumab: A Case Report and Review of the Literature. Case Rep Oncol, 10:407-415, 2017
24.Honma Y, Hokamura N, Nagashima K, Sudo K, Shoji H, Iwasa S, Takashima A, Kato K, Hamaguchi T, Boku N, Umezawa R, Ito Y, Itami J, Koyanagi K, Igaki H, Tachimori Y. Clinical Outcomes of Resectable Esophageal Cancer with Supraclavicular Lymph Node Metastases Treated with Curative Intent. Anticancer Res, 37:3741-3749, 2017
25.Kataoka K, Takeuchi H, Mizusawa J, Igaki H, Ozawa S, Abe T, Nakamura K, Kato K, Ando N, Kitagawa Y. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann Surg, 265:1152-1157, 2017
26.Nishikawa K, Takahashi T, Takaishi H, Miki A, Noshiro H, Yoshikawa T, Nishida Y, Iwasa S, Miwa H, Masuishi T, Boku N, Yamada Y, Kodera Y, Yoshida K, Morita S, Sakamoto J, Saji S, Kitagawa Y. Phase II study of the effectiveness and safety of trastuzumab and paclitaxel for taxane- and trastuzumab-naive patients with HER2-positive, previously treated, advanced, or recurrent gastric cancer (JFMC45-1102). Int J Cancer, 140:188-196, 2017
27.Fukuoka K, Nara S, Honma Y, Kishi Y, Esaki M, Shimada K. Hepatectomy for Colorectal Cancer Liver Metastases in the Era of Modern Preoperative Chemotherapy: Evaluation of Postoperative Complications. World J Surg, 41:1073-1081, 2017
28.Maniwa T, Mori K, Ohde Y, Okumura T, Boku N, Hishida T, Sakao Y, Yoshiya K, Hyodo I, Kondo H. Heterogeneity of Tumor Sizes in Multiple Pulmonary Metastases of Colorectal Cancer as a Prognostic Factor. Ann Thorac Surg, 103:254-260, 2017
29.Mizukami T, Sakai K, Naruki S, Taniyama T, Horie Y, Izawa N, Tsuda T, Fujino T, Boku N, Yasuda H, Fukunaga T, Nakajima TE, Nishio K. Identification of a FGFR3-TACC3 fusion in esophageal cancer. Ann Oncol, 28:437-438, 2017
30.Shiono S, Okumura T, Boku N, Hishida T, Ohde Y, Sakao Y, Yoshiya K, Hyodo I, Mori K, Kondo H. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg, 51:504-510, 2017
31.Hishida T, Tsuboi M, Okumura T, Boku N, Ohde Y, Sakao Y, Yoshiya K, Hyodo I, Mori K, Kondo H. Does Repeated Lung Resection Provide Long-Term Survival for Recurrent Pulmonary Metastases of Colorectal Cancer? Results of a Retrospective Japanese Multicenter Study. Ann Thorac Surg, 103:399-405, 2017
32.Nishimura T, Iwasa S, Nagashima K, Okita N, Takashima A, Honma Y, Kato K, Hamaguchi T, Yamada Y, Shimada Y, Boku N. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer, 20:655-662, 2017
33.Okuno T, Wakabayashi M, Kato K, Shinoda M, Katayama H, Igaki H, Tsubosa Y, Kojima T, Okabe H, Kimura Y, Kawano T, Kosugi S, Toh Y, Kato H, Nakamura K, Fukuda H, Ishikura S, Ando N, Kitagawa Y. Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303). Int J Clin Oncol, 22:1042-1049, 2017
34.Iizumi S, Takashima A, Narita Y, Tajika M, Muro K, Kawai S, Yasui H, Matsushima T, Takahari D, Nagashima K, Boku N. Efficacy and safety of taxane monotherapy in advanced gastric cancer refractory to triplet chemotherapy with docetaxel, cisplatin, and S-1: a multicenter retrospective study. Cancer Chemother Pharmacol, 80:575-582, 2017
35.Hagiwara Y, Ohashi Y, Okusaka T, Ueno H, Ioka T, Boku N, Egawa S, Hatori T, Furuse J, Mizumoto K, Ohkawa S, Yamaguchi T, Yamao K, Funakoshi A, Cheng AL, Kihara K, Sato A, Tanaka M. Health-related quality of life in a randomised phase III study of gemcitabine plus S-1, S-1 alone and gemcitabine alone for locally advanced or metastatic pancreatic cancer: GEST study. ESMO Open, 2:e000151, 2017
36.Katakami N, Harada T, Murata T, Shinozaki K, Tsutsumi M, Yokota T, Arai M, Tada Y, Narabayashi M, Boku N. Randomized Phase III and Extension Studies of Naldemedine in Patients With Opioid-Induced Constipation and Cancer. J Clin Oncol, 35:3859-3866, 2017
37.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet, 390:2461-2471, 2017
38.Bang YJ, Xu RH, Chin K, Lee KW, Park SH, Rha SY, Shen L, Qin S, Xu N, Im SA, Locker G, Rowe P, Shi X, Hodgson D, Liu YZ, Boku N. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol, 18:1637-1651, 2017
39.Sakamaki K, Kito Y, Yamazaki K, Izawa N, Tsuda T, Morita S, Boku N. Exploration of time points and cut-off values for early tumour shrinkage to predict survival outcomes of patients with metastatic colorectal cancer treated with first-line chemotherapy using a biexponential model for change in tumour size. ESMO Open, 2:e000275, 2017
40.Shoji H, Tada K, Kitano S, Nishimura T, Shimada Y, Nagashima K, Aoki K, Hiraoka N, Honma Y, Iwasa S, Takashima A, Kato K, Boku N, Honda K, Yamada T, Heike Y, Hamaguchi T. The peripheral immune status of granulocytic myeloid-derived suppressor cells correlates the survival in advanced gastric cancer patients receiving cisplatin-based chemotherapy. Oncotarget, 8:95083-95094, 2017
41.Ito Y, Tsuda T, Minatogawa H, Kano S, Sakamaki K, Ando M, Tsugawa K, Kojima Y, Furuya N, Matsuzaki K, Fukuda M, Sugae S, Ohta I, Arioka H, Tokuda Y, Narui K, Tsuboya A, Suda T, Morita S, Boku N, Yamanaka T, Nakajima TE. Placebo-Controlled, Double-Blinded Phase III Study Comparing Dexamethasone on Day 1 With Dexamethasone on Days 1 to 3 With Combined Neurokinin-1 Receptor Antagonist and Palonosetron in High-Emetogenic Chemotherapy. J Clin Oncol, 36:1000-1006, 2018
42.Hagiwara Y, Ohashi Y, Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T. Health-related quality of life of adjuvant chemotherapy with S-1 versus gemcitabine for resected pancreatic cancer: Results from a randomised phase III trial (JASPAC 01). Eur J Cancer, 93:79-88, 2018
43.Hasuike N, Ono H, Boku N, Mizusawa J, Takizawa K, Fukuda H, Oda I, Doyama H, Kaneko K, Hori S, Iishi H, Kurokawa Y, Muto M. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer, 21:114-123, 2018
44.Iizumi S, Takashima A, Sakamaki K, Morita S, Boku N. Survival impact of post-progression chemotherapy in advanced gastric cancer: systematic review and meta-analysis. Cancer Chemother Pharmacol, 81:981-989, 2018
45.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol, 23:1-34, 2018
46.Miura Y, Sukawa Y, Hironaka S, Mori M, Nishikawa K, Tokunaga S, Okuda H, Sakamoto T, Taku K, Moriwaki T, Negoro Y, Kimura Y, Uchino K, Shinozaki K, Shinozaki H, Musha N, Yoshiyama H, Tsuda T, Miyata Y, Sugimoto N, Shirakawa T, Ito M, Yonesaka K, Yoshimura K, Boku N, Nosho K, Takano T, Hyodo I. Five-weekly S-1 plus cisplatin therapy combined with trastuzumab therapy in HER2-positive gastric cancer: a phase II trial and biomarker study (WJOG7212G). Gastric Cancer, 21:84-95, 2018
47.Singh S, Carnaghi C, Buzzoni R, Pommier RF, Raderer M, Tomasek J, Lahner H, Valle JW, Voi M, Bubuteishvili-Pacaud L, Lincy J, Wolin E, Okita N, Libutti SK, Oh DY, Kulke M, Strosberg J, Yao JC, Pavel ME, Fazio N. Everolimus in Neuroendocrine Tumors of the Gastrointestinal Tract and Unknown Primary. Neuroendocrinology, 106:211-220, 2018
48.Sasaki Y, Iwasa S, Okazaki S, Goto M, Kojima Y, Naganuma A, Nagashima K, Nagai Y, Hirano H, Honma Y, Takashima A, Kato K, Hamaguchi T. A phase II study of combination therapy with oral S-1 and cisplatin in elderly patients with advanced gastric cancer. Gastric Cancer, 21:439-445, 2018
49.Nozaki I, Mizusawa J, Kato K, Igaki H, Ito Y, Daiko H, Yano M, Udagawa H, Nakagawa S, Takagi M, Kitagawa Y. Impact of laparoscopy on the prevention of pulmonary complications after thoracoscopic esophagectomy using data from JCOG0502: a prospective multicenter study. Surg Endosc, 32:651-659, 2018
50.Moriwaki T, Fukuoka S, Taniguchi H, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama C, Denda T, Satake H, Suto T, Sugimoto N, Enomoto M, Ishikawa T, Kashiwada T, Sugiyama M, Komatsu Y, Okuyama H, Baba E, Sakai D, Watanabe T, Tamura T, Yamashita K, Gosho M, Shimada Y. Propensity Score Analysis of Regorafenib Versus Trifluridine/Tipiracil in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapy (REGOTAS): A Japanese Society for Cancer of the Colon and Rectum Multicenter Observational Study. Oncologist, 23:7-15, 2018
51.Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, Nakao M, Sakai H, Nakayama T, Minato K, Arai T, Suzuki K, Shimada Y, Nagashima K, Terakado H, Yamamoto N. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol, 23:382-388, 2018
52.Sawaki A, Yamada Y, Yamaguchi K, Nishina T, Doi T, Satoh T, Chin K, Boku N, Omuro Y, Komatsu Y, Hamamoto Y, Koizumi W, Saji S, Shah MA, Van Cutsem E, Kang YK, Iwasaki J, Kuriki H, Ohtsuka W, Ohtsu A. Regional differences in advanced gastric cancer: exploratory analyses of the AVAGAST placebo arm. Gastric Cancer, 21:429-438, 2018
53.Sudo K, Kato K, Kuwabara H, Sasaki Y, Takahashi N, Shoji H, Iwasa S, Honma Y, Okita NT, Takashima A, Hamaguchi T, Yamada Y, Ito Y, Itami J, Fukuda T, Tobinai K, Boku N. Patterns of Relapse after Definitive Chemoradiotherapy in Stage II/III (Non-T4) Esophageal Squamous Cell Carcinoma. Oncology, 94:47-54, 2018
54.Akiyoshi K, Hamaguchi T, Yoshimura K, Takahashi N, Honma Y, Iwasa S, Takashima A, Kato K, Yamada Y, Onodera H, Takeshita S, Yasui H, Sakai G, Akatsuka S, Ogawa K, Horita Y, Nagai Y, Shimada Y. A Prospective, Multicenter Phase II Study of the Efficacy and Feasibility of 15-minute Panitumumab Infusion Plus Irinotecan for Oxaliplatin- and Irinotecan-refractory, KRAS Wild-type Metastatic Colorectal Cancer (Short Infusion of Panitumumab Trial). Clin Colorectal Cancer, 17:e83-e89, 2018
55.Kusumoto T, Sunami E, Ota M, Yoshida K, Sakamoto Y, Tomita N, Maeda A, Mochizuki I, Okabe M, Kunieda K, Yamauchi J, Itabashi M, Kotake K, Takahashi K, Baba H, Boku N, Aiba K, Ishiguro M, Morita S, Sugihara K. Planned Safety Analysis of the ACTS-CC 02 Trial: A Randomized Phase III Trial of S-1 With Oxaliplatin Versus Tegafur and Uracil With Leucovorin as Adjuvant Chemotherapy for High-Risk Stage III Colon Cancer. Clin Colorectal Cancer, 17:e153-e161, 2018
56.Kato K, Ura T, Koizumi W, Iwasa S, Katada C, Azuma M, Ishikura S, Nakao Y, Onuma H, Muro K. Nimotuzumab combined with concurrent chemoradiotherapy in Japanese patients with esophageal cancer: A phase I study. Cancer Sci, 109:785-793, 2018
57.Hiramoto S, Kato K, Shoji H, Okita N, Takashima A, Honma Y, Iwasa S, Hamaguchi T, Yamada Y, Shimada Y, Boku N. A retrospective analysis of 5-fluorouracil plus cisplatin as first-line chemotherapy in the recent treatment strategy for patients with metastatic or recurrent esophageal squamous cell carcinoma. Int J Clin Oncol, 23:466-472, 2018
