Annual Report 2017
Department of Dermatologic Oncology
Naoya Yamazaki, Arata Tsutsumida, Akira Takahashi, Kenjiro Namikawa
Introduction
The Department of Dermatologic Oncology has consistently served as the core hospital for the establishment of treatment strategies for malignant skin tumors since the opening of the National Cancer Center (NCC) in 1962, with over 3,000 cases of malignant melanoma treated thus far; an impressive number for a hospital or research institution in Japan. Today, patients are referred from all over Japan. Particularly noteworthy is the total of 202 patients with malignant melanoma, approximately double the number of ten years ago. Most of patients are examined and treated for skin cancer including malignant melanoma. Surgery is the main treatment modality for skin cancer, while multi-disciplinary treatments, comprising of immunotherapy, targeted therapy, chemotherapy, and radiotherapy, are also routinely carried out. But the importance of immunotherapy is especially increased these days. Melanoma is at the center of development of the immune therapy. This department plays an active role in multicenter trials of new agents for skin cancer all over Japan.
Routine activities
The Department of Dermatologic Oncology has four staff dermatologic oncologists, and four residents. We are also engaged in routine outpatient activities on Wednesdays and Thursdays in the NCC Hospital East (NCCHE).
Our department is a high-volume center, where we have seen an average of 180 patients with malignant melanoma annually for the past five years. This follows the establishement of a national network to develop treatment for malignant skin tumors, and nivolumab, an anti-PD-1 antibody, was approved as a therapeutic agent for malignant melanoma in Japan as a world first and reflecting vigorous new drug development.
About 20 patients are hospitalized to undergo surgery, chemotherapy, or radiation therapy. In 2017, 339 operations were performed, including 150 under general anesthesia.
For first-line systemic therapy of unresectable or metastatic melanoma, recommended treatment options include checkpoint immunotherapy, BRAF-targeted therapy for patients with BRAF-mutated disease, or clinical trial.
Checkpoint immunotherapy options in this setting include anti-PD-1 monotherapy with pembrolizumab or nivolumab. Checkpoint inhibitors have been shown to be effective regardless of BRAF-mutation status.
For most patients with BRAF-mutant metastatic disease, BRAF-targeted therapy first-line options include BRAF inhibitor mono therapy with vemrafenib or BRAF/MEK inhibitor combination therapy with dabrafenib/trametinib.
Rounds are made and case presentations are held every morning. A department conference is also held every Monday to discuss the therapeutic principles for outpatients and inpatients. A clinicopathological conference focusing on surgically removed skin specimens is held with pathologists once a month.
Furthermore, we have treated patients with advanced mucosal melanoma in the nasal cavity, genital lesions, perianal lesions, and uveal melanoma, despite our original "dermatologic" specialty.
Research activities
Malignant skin tumors are mainly treated by surgery (appended table). However, in recent years, several new drugs have been rapidly developed overseas for the treatment of malignant melanoma, and our department has been conducting numerous clinical studies and trials; the most important ones are described below.
*A multicenter study to establish the standard therapy for refractory malignancies
*A study on the establishment of an early clinical development system of drugs for rare cancers and support for research
*Development of a system for boron neutron capture therapy (BNCT) using an accelerator installed at the hospital
*An atlas and management of skin toxicities associated with anticancer agents
*A study on methods for assessing skin changes associated with cancer treatment and the establishment of standard care
*A study on the quantitative assessment of skin disorders associated with chemotherapy using molecular-targeted agents and skin care
*A retrospective study to clarify the outcomes of conventional treatment for cutaneous angiosarcoma of the head and neck
*A retrospective study on the outcomes of TACE therapy using cisplatin for liver metastasis from primary ocular malignant melanoma
*A phase I/II trial of combined dabrafenib and trametinib in patients with BRAF V600E or V600K mutation-positive advanced solid cancer (for phase I trial) or cutaneous malignant melanoma (for phase II trial)
*A randomized double-blind Phase III study comparing placebo and combination therapy with dabrafenib (GSK2118436) and trametinib (GSK1120212), given as postoperative adjuvant therapy for BRAF V600 mutation-positive malignant melanoma (high-risk group for recurrence)
*A phase II, open-label, multicenter study to evaluate the efficacy and safety of avelumab (MSB0010718C) in patients with Merkel cell carcinoma
*A Phase II study of combination treatment with canerpaturev (HF10), an oncolytic viral immunotherapy, and ipilimumab in patients with unresectable or metastatic melanoma after anti-
PD-1 therapy
*A working group to prepare guidelines on "Anti-immune checkpoint therapy and combination therapy" or leaflets explaining the guidelines
*"Development of innovative cancer immunotherapy by identifying essential aspects of the tumor microenvironment associated with malignant melanoma"
*Clinical evaluation of a practical, non-invasive diagnostic tool for superficial skin tumors (hyperspectral imager)
*Practical development of therapeutic agents for refractory skin cancer using innovative
molecular-targeted agents inducing cancer-
specific apoptosis through an investigator-
initiated clinical trial
Clinical trials
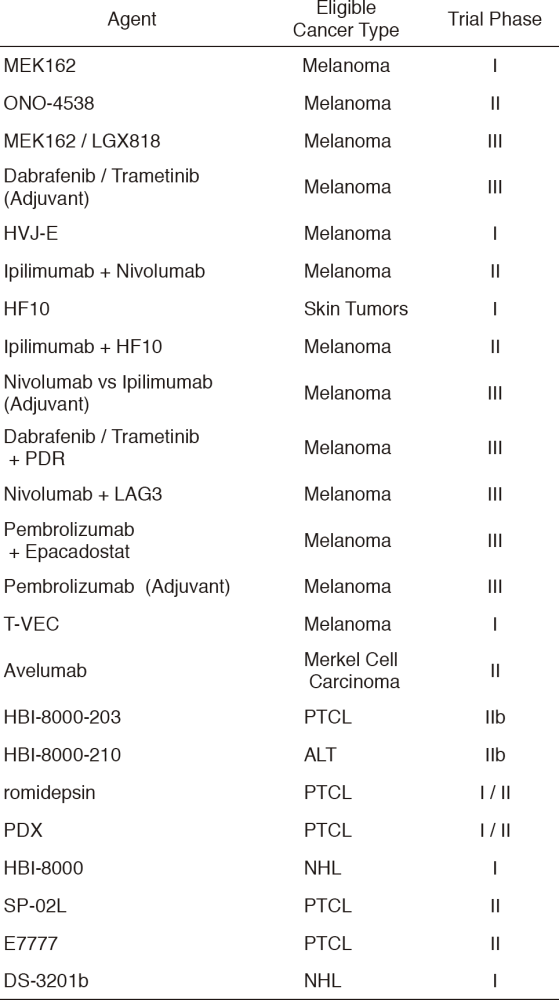
Table 3 shows our clinical trials.
Education
Currently, six resident physicians are engaged in ongoing training in routine clinical practice under skilled guidance. In addition, conferences with the Departments of Medical Oncology, Radiation Oncology, Pathology, and the Division of Cancer Immunology of the NCC Research Institute (NCCRI) are held on a regular basis. The resident physicians and the oncology trainee made a total of 10 presentations in domestic academic conferences as well as publishing two papers, one of them was an international academic paper in English.
Future prospects
We have devised certain measures to resolve the drug lag between Japan and Western countries in the treatment of malignant melanomas and will further promote the development of effective and safe treatment strategies.
Our department is a high-volume center in Japan for malignant melanomas and other malignant skin tumors. With the advantageous data collection associated with such a large patient base, we will further strengthen our research collaboration system even more than at present, leveraging a translational research platform with the NCCRI.
Our department is trying to participate in international randomized controlled trials as a top team of all Asian dermatologic oncology teams every time.
Table 1-1. Number of New Patients (2001-2009)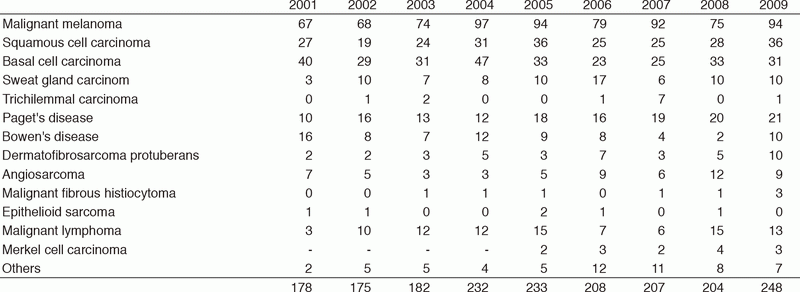
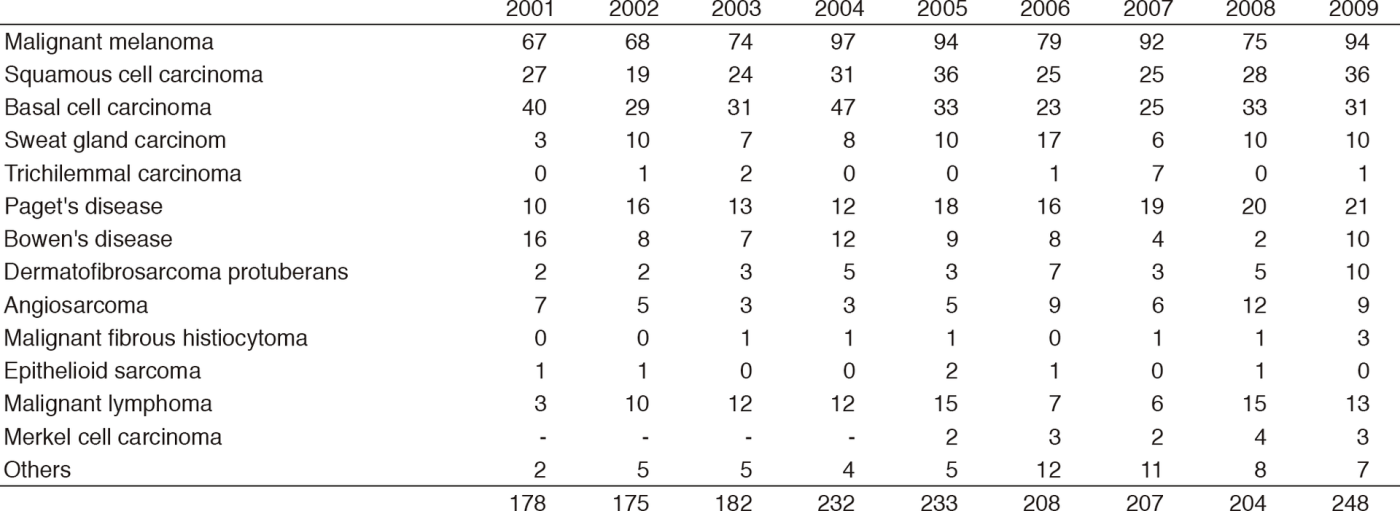
Table 1-2. Number of New Patients (2010-2018/3)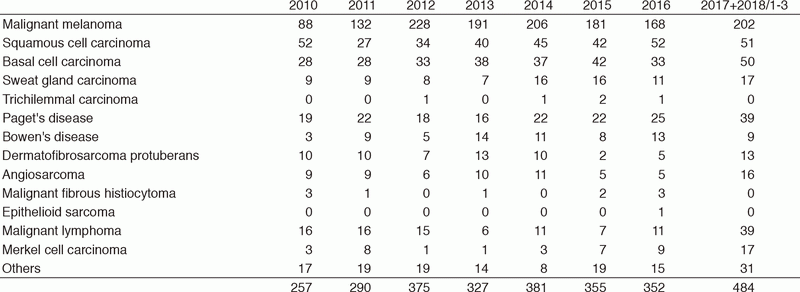
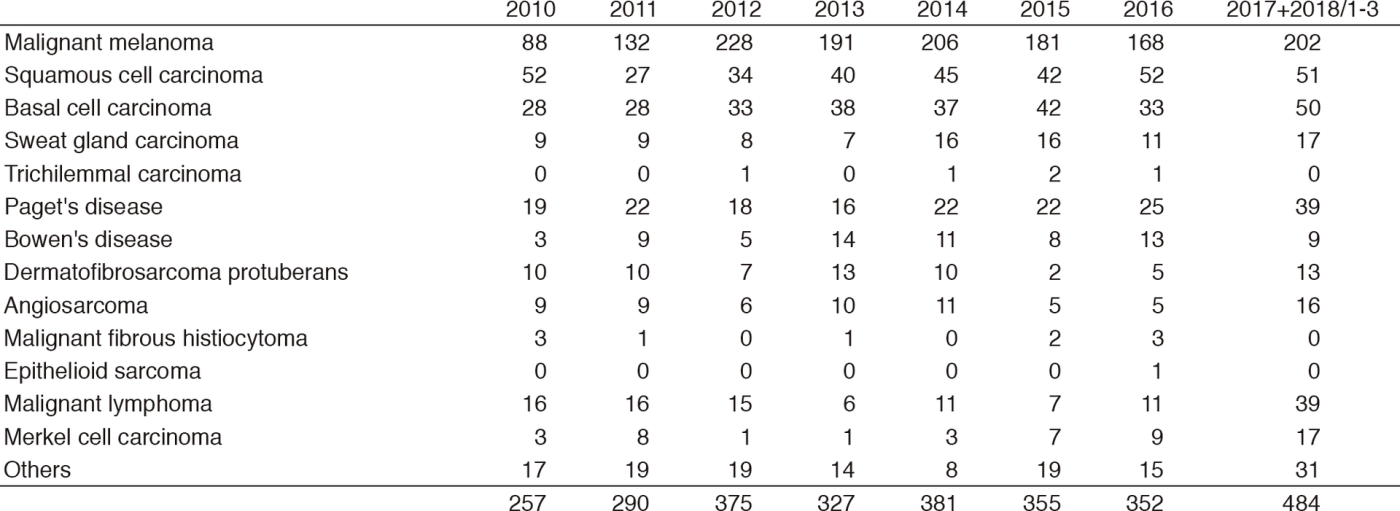
Table 2. Operative Procedures (total number) in January 2017 - March 2018

Table 3. New Agent Studies in January 2017 - March 2018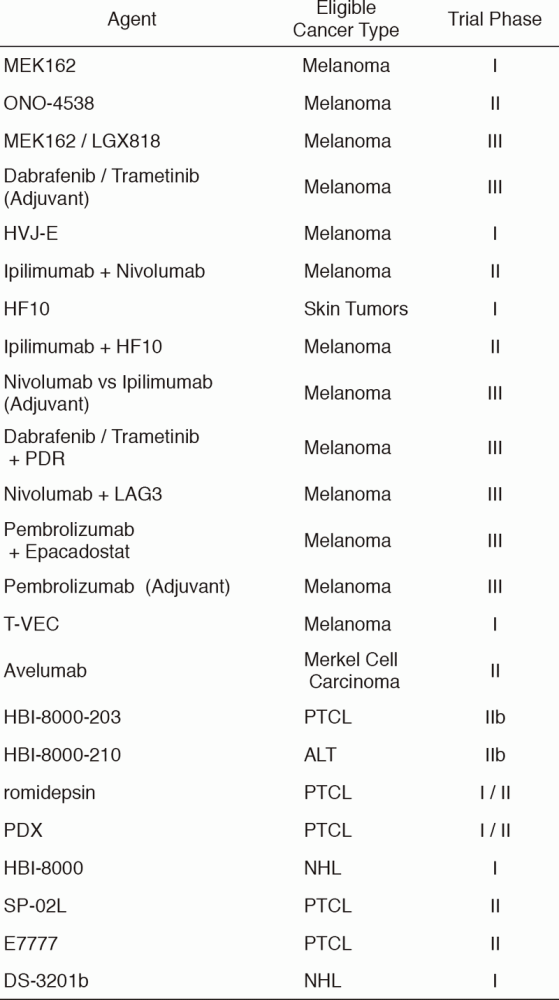
List of papers published in January 2017 - March 2018
Journal
1. Tomizuka T, Namikawa K, Higashi T. Characteristics of melanoma in Japan: a nationwide registry analysis 2011-2013. Melanoma Res, 27:492-497, 2017
2. Yamazaki N, Kiyohara Y, Uhara H, Iizuka H, Uehara J, Otsuka F, Fujisawa Y, Takenouchi T, Isei T, Iwatsuki K, Uchi H, Ihn H, Minami H, Tahara H. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci, 108:1022-1031, 2017
3. Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T, Kiyohara Y, Uhara H, Nakagawa K, Furukawa H, Wada H, Noguchi K, Shimamoto T, Yokota K. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemother Pharmacol, 79:651-660, 2017
4. Namikawa K, Tsutsumida A, Mizutani T, Shibata T, Takenouchi T, Yoshikawa S, Kiyohara Y, Uchi H, Furue M, Ogata D, Tsuchida T, Yamazaki N. Randomized phase III trial of adjuvant therapy with locoregional interferon beta versus surgery alone in stage II/III cutaneous melanoma: Japan Clinical Oncology Group Study (JCOG1309, J-FERON). Jpn J Clin Oncol, 47:664-667, 2017
5. Ogata D, Uhara H, Tsutsumida A, Yamazaki N, Mochida K, Amano M, Yoshikawa S, Kiyohara Y, Tsuchida T. Nail apparatus melanoma in a Japanese population: a comparative study of surgical procedures and prognoses in a large series of 151 cases. Eur J Dermatol, 27:620-626, 2017
6. Yamazaki N, Kiyohara Y, Uhara H, Uehara J, Fujimoto M, Takenouchi T, Otsuka M, Uchi H, Ihn H, Minami H. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: A phase II study. Cancer Sci, 108:1223-1230, 2017
7. Nakamura Y, Mori T, Takae Y, Tsutsumida A, Takahashi A, Namikawa K, Hirai I, Yamazaki N. Rapidly developed BRAF inhibitor-induced verrucous keratosis. J Dermatol, 44:e274-e275, 2017
8. Omata W, Tsutsumida A, Namikawa K, Takahashi A, Oashi K, Yamazaki N. Sequential Combination Chemotherapy of Dacarbazine (DTIC) with Carboplatin and Paclitaxel for Patients with Metastatic Mucosal Melanoma of Nasal Cavity and Paranasal Sinuses. Clin Med Insights Case Rep, 10:1-5, 2017
9. Tsuneki M, Kinjo T, Mori T, Yoshida A, Kuyama K, Ohira A, Miyagi T, Takahashi K, Kawai A, Chuman H, Yamazaki N, Masuzawa M, Arakawa H. Survivin: A novel marker and potential therapeutic target for human angiosarcoma. Cancer Sci, 108:2295-2305, 2017
10. Ng W, Takahashi A, Muto Y, Yamazaki N. High-risk cutaneous squamous cell carcinoma in a Japanese allogeneic bone marrow transplant recipient on long-term voriconazole. J Dermatol, 44:1152-1155, 2017
11. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob JJ, Butler MO, Middleton MR, Maio M, Atkinson V, Queirolo P, Gonzalez R, Kudchadkar RR, Smylie M, Meyer N, Mortier L, Atkins MB, Long GV, Bhatia S, Lebbe C, Rutkowski P, Yokota K, Yamazaki N, Kim TM, de Pril V, Sabater J, Qureshi A, Larkin J, Ascierto PA. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med, 377:1824-1835, 2017
12. Shibayama Y, Namikawa K, Sone M, Takahashi A, Tsutsumida A, Sugawara S, Arai Y, Aihara Y, Suzuki S, Nakayama J, Imafuku S, Yamazaki N. Efficacy and toxicity of transarterial chemoembolization therapy using cisplatin and gelatin sponge in patients with liver metastases from uveal melanoma in an Asian population. Int J Clin Oncol, 22:577-584, 2017
13. Chang JW, Guo J, Hung CY, Lu S, Shin SJ, Quek R, Ying A, Ho GF, Nguyen HS, Dhabhar B, Sriuranpong V, Tiambeng ML, Prayogo N, Yamazaki N. Sunrise in melanoma management: Time to focus on melanoma burden in Asia. Asia Pac J Clin Oncol, 13:423-427, 2017
14. Uhara H, Kiyohara Y, Tsuda A, Takata M, Yamazaki N. Characteristics of adverse drug reactions in a vemurafenib early post-marketing phase vigilance study in Japan. Clin Transl Oncol, 20:169-175, 2018
15. Namikawa K, Aung PP, Gershenwald JE, Milton DR, Prieto VG. Clinical impact of ulceration width, lymphovascular invasion, microscopic satellitosis, perineural invasion, and mitotic rate in patients undergoing sentinel lymph node biopsy for cutaneous melanoma: a retrospective observational study at a comprehensive cancer center. Cancer Med, 7:583-593, 2018
16. Fujiwara Y, Yamazaki N, Kiyohara Y, Yoshikawa S, Yamamoto N, Tsutsumida A, Nokihara H, Namikawa K, Mukaiyama A, Zhang F, Tamura T. Safety, tolerability, and pharmacokinetic profile of dabrafenib in Japanese patients with BRAF (V600) mutation-positive solid tumors: a phase 1 study. Invest New Drugs, 36:259-268, 2018
