Annual Report 2017
Clinical Research Support Office
Yasuhiro Fujiwara
- Clinical Research Coordinating Division
Noboru Yamamoto, Hiroko Nakahama, Miki Ito, Noriko Kobayashi, Yukako Takasaki, Katsuyuki Ikarashi, Mari Takahashi, Sho Murata, Keiko Igarashi, Asako Sakamoto, Kumiko Hirayama, Tomomi Tsuchiya, Yoshimi Yamaguchi, Kazumi Matsuo, Kikue Kamiyama, Harue Ui, Shino Osawa, Tamami Yamano, Chie Miyano, Hiroko Kawaguchi, Noriko Makita, Yuko Tanoue, Yukari Nishiyama, Saki Yoshizawa, Ai Sekido, Yukari Torihata, Emi Yasuda, Ikuko Mitsuishi, Hiroko Minami, Shinobu Araki, Setsuko Kamizaki, Ran Obara, Mikiko Sega, Chie Moteki, Ami Hashimoto, Ayako Kuwabara, Mari Kondo, Yumiko Ikuno, Hatuki Ono, Azumi Seko, Kiyoka Ishihama, Mai Koda, Nobuko Ushirozawa, Yoko Ebihara, Harumi Maruno, Jyunko Horie, Kaori Matsushita, Tsukina Soku, Eiko Mitsumata, Yuka Higashi, Ken Kato, Keiko Wakakuwa, Risa Miyagawa, Keiko Shimo, Harumi Mochizuki, Satomi Nakamori, Yuki Sako, Mayumi Ikeda - Research Management Division
Kenichi Nakamura, Ayaka Ohmine, Tomomi Hata, Takashi Hamada, Makiko Watanabe, Kozo Kataoka, Mitsumi Terada, Natsuko Okita, Tamie Sukigara, Mamiko Kawasaki, Akio Tsuboshita, Hitomi Ohkuma, Tsubasa Nagata, Akane Matsushima, Hiroshi Katayama, Eiko Yorikane, Sawako Tomatsuri, Ritsuko Nagasaka, Sachie Kawabata, Hidekazu, Saito, Junko Eba, Keisuke Kanato, Akiko Noguchi, Kenichi Miyamoto, Kiyo Tanaka, Tomohiro Kadota, Yuya Sato, Taro Shibata, Aya Kuchiba, Junki Mizusawa, Gakuto Ogawa, Akihiro Hirakawa, Yasue Kojima, Kan Yonemori, Satoshi Kawashima - Data Management Division
Haruhiko Fukuda, Harumi Kaba, Tomoko Kusakabe, Mikio Mori, Nobuko Okamura, Ryuji Makiuchi, Rie Nagumo, Chiho Shimojima, Yukari Hoshina, Kaoru Koike, Keiko Ohata, Tomoaki Yamada, Masahisa Kamikura, Yukari Nagasaka, Keiko Suto
Introduction
The Clinical Research Support Office supports clinical research conducted under the leadership by investigators in the National Cancer Center Hospital (NCCH). Supporting activities include protocol writing, central/local data management, statistical design and analysis, in-house/on-site monitoring, audits, patient recruitment, and other coordinating jobs.
Our team and what we do
1. Clinical Research Coordinating Division
The Clinical Research Coordinating Section and the Clinical Trial Administration Section support a lot of industry-sponsored registration trials as well as the physician-initiated registration-directed clinical trials. A total of 34 CRCs (clinical research coordinators), seven CRC assistants, and seven administration staff are supporting these trials.
The Biobank and Translational Research Support Section has routinely obtained the informed consent to participate as an NCC biobank (NCCBB) donor from patients who consult with the NCCH for the first time. CRCs in this section coordinate the translational research in several ways, such as assistance of registration for clinical trials, logistics of pathological specimens, data collection for case report forms, and coordination between sections. We explained the purport of the NCCBB to 8,781 patients from April 2017 to March 2018, and received consent for blood collection and research use of their surplus samples for research from 8,508 patients (96.9% consent proportion). The patient load with our assistance in filling in the preliminary-diagnosis card and so on was 9,716. We also support eight biomarker trials, and for registered patients (pts), five pts for BT-SCRUM, eight pts for PRELUDE study, 53 pts for TOP-GEAR SciLabo, 22 pts for MASTER KEY project, 85 pts for micro NRA project, nine pts for GI-SCREEN_CRC study, and 14 pts for GI-SCREEN_non CRC study.
2. Research Management Division
The Research Management Division is in charge of central research support functions: i) International clinical trial management; ii) Investigational new drug (IND) trial management; iii) Monitoring & Consultation; iv) Multi-institutional clinical trial support; v) Biostatistics;and vi) Pharmaceutical affairs consultation. One of the strengths of the NCCH is implementing various types of clinical trials covering both early phase trials including first-in-human trials and late phase multi-institutional trials. The IND trial management function is responsible for comprehensive study coordination and site visit monitoring in early phase trials. The multi-institutional trial support function works as the Japan Clinical Oncology Group (JCOG) Operations Office which engages in protocol development, manuscript drafting, study coordination, etc. for late phase trials.
3. Data Management Division
The Data Management Division is responsible for central data management and in-house study monitoring in investigator-initiated clinical trials for cancer therapeutic development. The Data Management Section supports early phase cancer trials mainly for drug development including registration trials which are led by physicians in the NCCH. The Multi-institutional Data Management Section supports mostly late development multi-modality multi-institutional phase II or phase III trials for adult solid cancer conducted by the JCOG.
Research activities
Research Management Division
At academic conferences, several presentations were made by section members such as:
i) NCCH initiative as a Clinical Research Core Hospital; ii) Risk-based monitoring in the NCCH; and iii) Utilization of Central IRB in multi-institutional clinical trials.
Clinical trials
1. Clinical Research Coordinating Division
The number of industry-sponsored registration trials is increasing year by year, and the division supported 365 registration-directed clinical trials including 27 investigator-initiated registration directed trials in FY 2017 (Table 1).
2. Research Management Division
As of the end of 2017, the Research Management Division supported 17 IND trials, and three clinical trials using Advanced Medical Care system. This division has been in charge of the coordinating office of rare cancer registry study with basket sub-studies since its start in April 2017 (MASTER KEY Project). This division also supports an international investigator-initiated registration-directed trial (PATHWAY trial). The number of study planning consultation was 33 and the number of studies reviewed in the concept review committee was 26.
3. Data Management Division
The Data Management Section supports 19 IND trials (six open, eight in preparation, five on follow-up) and 14 non-IND studies (10 open, three in preparation, one on follow-up). The Multi-institutional Data Management Section supports 87 non-IND trials (54 open, 10 in preparation, 23 on follow-up) as the JCOG Data Center.
Education
The staff members received not only day-to-day on-the-job training but also in-house educational seminars and the JCOG internal educational programs in order to learn clinical trial methodology, project management, monitoring, and research ethics.
Future prospects
1. Clinical Research Coordinating Division
The number of supported clinical trials is increasing as previously described, and the supporting area covered by CRCs will be expanded to include not only registration trials but also other investigator-initiated clinical trials. Therefore, the expansion of CRC staff members is highly anticipated. In view of the plan for the NCCH, all members of this office will work together to contribute to reinforcing clinical research capabilities of the NCCH and to making this office a valuable unit for all members of our hospital.
The member of Biobank and Translational Research Support Section received seminars which are related to clinical research ethics, etc. The section informs and educates investigators of the NCC about the NCC biobank and translational research periodically through the NCC University. As a future direction, the section proceeds improvement of quality of the NCC biobank about informatics and storage of serum. And the section aims to establish a support system for higher quality and quantity.
2. Research Management Division
Since the number of IND trials has increased from 11 in 2016 to 17 trials in 2017, reinforcement of staff resources is urgently needed. Since 2017, the Research Management Division has changed the system to make a profit by study support expenses and the budget of the division has substantially increased. In response to this increase, this division will reinforce the support function for various clinical trials such as international clinical trials, IND trials, and advanced medical care system trials. Also, this division will establish optimal ways to cope with the newly enacted Clinical Research Act.
3. Data Management Division
The Data Management Division is introducing web-based electronic data capturing (EDC) system and is promoting standardization of all aspects of data management, such as data format, case report forms and monitoring reports for increasing data integrity, and cost effectiveness of day-to-day works.
Table 1. Supported Trials in Clinical Research Coordinating Division in FY 2017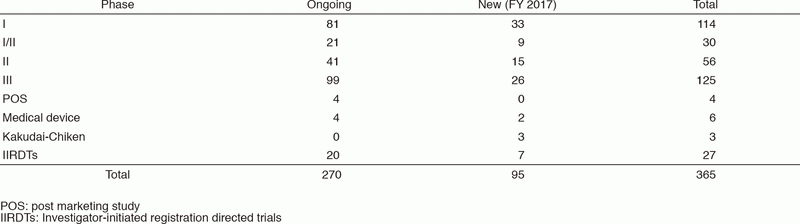
List of papers published in January 2017 - March 2018
Journal
1. Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg, 265:277-283, 2017
2. Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A, Nakamori M, Onaya H, Sasako M. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric cancer, 20:322-331, 2017
3. Tsuruoka K, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Asakura K, Nakagawa K, Sakurai H, Watanabe SI, Tsuta K, Ohe Y. PD-L1 expression in neuroendocrine tumors of the lung. Lung Cancer, 108:115-120, 2017
4. Sekine I, Harada H, Yamamoto N, Wakabayashi M, Murakami H, Goto K, Nogami N, Seto T, Oshita F, Okamoto H, Tanaka H, Tamura T, Ishikura S, Ohe Y. Randomized phase II trial of weekly dose-intensive chemotherapy or amrubicin plus cisplatin chemotherapy following induction chemoradiotherapy for limited-disease small cell lung cancer (JCOG1011). Lung Cancer, 108:232-237, 2017
5. Aokage K, Saji H, Suzuki K, Mizutani T, Katayama H, Shibata T, Watanabe S, Asamura H. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg, 65: 267-272, 2017
6. Kurokawa Y, Yamaguchi T, Sasako M, Sano T, Mizusawa J, Nakamura K, Fukuda H. Institutional variation in short- and long-term outcomes after surgery for gastric or esophagogastric junction adenocarcinoma: correlative study of two randomized phase III trials (JCOG9501 and JCOG9502). Gastric cancer, 20:508-516, 2017
7. Ohue M, Iwasa S, Kanemitsu Y, Hamaguchi T, Shiozawa M, Ito M, Yasui M, Katayama H, Mizusawa J, Shimada Y. A Phase II/III randomized controlled trial comparing perioperative versus postoperative chemotherapy with mFOLFOX6 for lower rectal cancer with suspected lateral pelvic node metastasis: Japan Clinical Oncology Group Study JCOG1310 (PRECIOUS study). Jpn J Clin Oncol, 47:84-87, 2017
8. Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Bandou H, Katsumata K, Murata K, Akagi Y, Takiguchi N, Saida Y, Nakamura K, Fukuda H, Akasu T, Moriya Y. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg, 266:201-207, 2017
9. Shimomura A, Kondo S, Kobayashi N, Iwasa S, Kitano S, Tamura K, Fujiwara Y, Yamamoto N. Do all patients in the phase I oncology trials need to be hospitalized? Domestic but outstanding issues for globalization of drug development in Japan. Int J Clin Oncol, 22:780-785, 2017
10. Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric cancer, 20:699-708, 2017
11. Namikawa K, Tsutsumida A, Mizutani T, Shibata T, Takenouchi T, Yoshikawa S, Kiyohara Y, Uchi H, Furue M, Ogata D, Tsuchida T, Yamazaki N. Randomized phase III trial of adjuvant therapy with locoregional interferon beta versus surgery alone in stage II/III cutaneous melanoma: Japan Clinical Oncology Group Study (JCOG1309, J-FERON). Jpn J Clin Oncol, 47:664-667, 2017
12. Nomura M, Kato K, Ando N, Ohtsu A, Muro K, Igaki H, Abe T, Takeuchi H, Daiko H, Gotoh M, Kataoka K, Wakabayashi M, Kitagawa Y. Comparison between neoadjuvant chemotherapy followed by surgery and definitive chemoradiotherapy for overall survival in patients with clinical Stage II/III esophageal squamous cell carcinoma (JCOG1406-A). Jpn J Clin Oncol, 47:480-486, 2017
13. Okuno T, Wakabayashi M, Kato K, Shinoda M, Katayama H, Igaki H, Tsubosa Y, Kojima T, Okabe H, Kimura Y, Kawano T, Kosugi S, Toh Y, Kato H, Nakamura K, Fukuda H, Ishikura S, Ando N, Kitagawa Y. Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303). Int J Clin Oncol, 22:1042-1049, 2017
14. Konagai A, Yoshimura K, Hazama S, Yamamoto N, Aoki K, Ueno T, Fujioka M, Iijima H, Kato M, Uchida M, Wada T, Inoue M, Asao T, Fuse M, Wada S, Kuramasu A, Kamei R, Takeda S, Yamamoto S, Yoshino S, Oka M, Nagano H. Correlation Between NKG2DL Expression and Antitumor Effect of Protein-bound Polysaccharide-K in Tumor-bearing Mouse Models. Anticancer Res, 37:4093-4101, 2017
15. Yamamoto N, Fujiwara Y, Tamura K, Kondo S, Iwasa S, Tanabe Y, Horiike A, Yanagitani N, Kitazono S, Inatani M, Tanaka J, Nishio M. Phase Ia/Ib study of the pan-class I PI3K inhibitor pictilisib (GDC-0941) administered as a single agent in Japanese patients with solid tumors and in combination in Japanese patients with non-squamous non-small cell lung cancer. Invest New Drugs, 35:37-46, 2017
16. Yamamoto N, Goto K, Nishio M, Chikamori K, Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T, Tajima K, Tamura T. Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol, 22:70-78, 2017
17. Kataoka K, Takeuchi H, Mizusawa J, Igaki H, Ozawa S, Abe T, Nakamura K, Kato K, Ando N, Kitagawa Y. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann Surg, 265:1152-1157, 2017
18. Kitano S, Inomata M, Mizusawa J, Katayama H, Watanabe M, Yamamoto S, Ito M, Saito S, Fujii S, Konishi F, Saida Y, Hasegawa H, Akagi T, Sugihara K, Yamaguchi T, Masaki T, Fukunaga Y, Murata K, Okajima M, Moriya Y, Shimada Y. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. The lancet. Gastroenterology & hepatology, 2:261-268, 2017
19. Onimaru R, Onishi H, Shibata T, Hiraoka M, Ishikura S, Karasawa K, Matsuo Y, Kokubo M, Shioyama Y, Matsushita H, Ito Y, Shirato H. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer (JCOG0702): Results for the group with PTV100cc. Radiother Oncol, 122:281-285, 2017
20. Asao T, Fujiwara Y, Itahashi K, Kitahara S, Goto Y, Horinouchi H, Kanda S, Nokihara H, Yamamoto N, Takahashi K, Ohe Y. Sequential Use of Anaplastic Lymphoma Kinase Inhibitors in Japanese Patients With ALK-Rearranged Non-Small-Cell Lung Cancer: A Retrospective Analysis. Clin Lung Cancer, 18:e251-e258, 2017
21. Okuma HS, Horinouchi H, Kitahara S, Asao T, Sunami K, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ohe Y. Comparison of Amrubicin and Weekly Cisplatin/Etoposide/Irinotecan in Patients With Relapsed Small-cell Lung Cancer. Clin Lung Cancer, 18:234-240 e2, 2017
22. Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y, Honda K, Tanabe Y, Wakui H, Tamura T. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs, 35:207-216, 2017
23. Fujiwara Y, Goto Y, Kanda S, Horinouchi H, Yamamoto N, Sakiyama N, Ando Makihara R, Ohe Y. Efficacy and safety of osimertinib in a Japanese compassionate use program. Jpn J Clin Oncol, 47:625-629, 2017
24. Kimura T, Nagata Y, Eba J, Ozawa S, Ishikura S, Shibata T, Ito Y, Hiraoka M, Nishimura Y. A randomized Phase III trial of comparing two dose-fractionations stereotactic body radiotherapy (SBRT) for medically inoperable Stage IA non-small cell lung cancer or small lung lesions clinically diagnosed as primary lung cancer: Japan Clinical Oncology Group Study JCOG1408 (J-SBRT trial). Jpn J Clin Oncol, 2017
25. Nomura S, Hirakawa A, Hamada C. Sample size determination for the current strategy in oncology Phase 3 trials that tests progression-free survival and overall survival in a two-stage design framework. J Biopharm Stat, 1-23, 2017
26. Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med, 5:42-50, 2017
27. Kubo E, Yamamoto N, Nokihara H, Fujiwara Y, Horinouchi H, Kanda S, Goto Y, Ohe Y. Randomized phase II study of sequential carboplatin plus paclitaxel and gefitinib in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: Long-term follow-up results. Molecular and clinical oncology, 6:56-62, 2017
28. Inokuchi J, Kuroiwa K, Kakehi Y, Sugimoto M, Tanigawa T, Fujimoto H, Gotoh M, Masumori N, Ogawa O, Eto M, Ohyama C, Yamaguchi A, Matsuyama H, Ichikawa T, Asano T, Mizusawa J, Eba J, Naito S. Role of lymph node dissection during radical nephroureterectomy for upper urinary tract urothelial cancer: multi-institutional large retrospective study JCOG1110A. World J Urol, 35:1737-1744, 2017
29. Sasada S, Ushirozawa N, Kobayashi N, Fujiwara Y, Tamura K, Yamamoto N. Surveillance of protocol deviations in Japanese oncology registration trials: a single institute experience. Invest New Drugs, 35:392-396, 2017
30. Nokihara H, Yamamoto N, Yamada Y, Honda K, Asahina H, Tamura Y, Hozak RR, Gao L, Suzukawa K, Enatsu S, Tamura T. A phase 1 study of ramucirumab in Japanese patients with advanced solid tumors. Jpn J Clin Oncol, 47:298-305, 2017
31. Kataoka K, Nakamura K, Caballero C, Evrard S, Negrouk A, Shiozawa M, Collette L, Fukuda H, Lacombe D. Collaboration between EORTC and JCOG-how to accelerate global clinical research partnership. Jpn J Clin Oncol, 47:164-169, 2017
32. Watari H, Katayama H, Shibata T, Ushijima K, Satoh T, Onda T, Aoki D, Fukuda H, Yaegashi N, Sakuragi N. Phase III trial to confirm the superiority of pelvic and para-aortic lymphadenectomy to pelvic lymphadenectomy alone for endometrial cancer: Japan Clinical Oncology Group Study 1412 (SEPAL-P3). Jpn J Clin Oncol, 47:986-990, 2017
33. Nakamichi S, Horinouchi H, Asao T, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ito Y, Watanabe SI, Ohe Y. Comparison of Radiotherapy and Chemoradiotherapy for Locoregional Recurrence of Non-small-cell Lung Cancer Developing After Surgery. Clin Lung Cancer, 18:e441-e448, 2017
