Annual Report 2017
Division of Cancer Biology
Hirofumi Arakawa, Yasuyuki Nakamura, Akiko Kuma, Masayuki Tsuneki, Yoko Sagami, Katsuko Honjo, Makoto Yamamoto, Katsuya Chinen, Naoki Tsukimata
Research activities
1. Mieap-regulated mitochondrial quality control
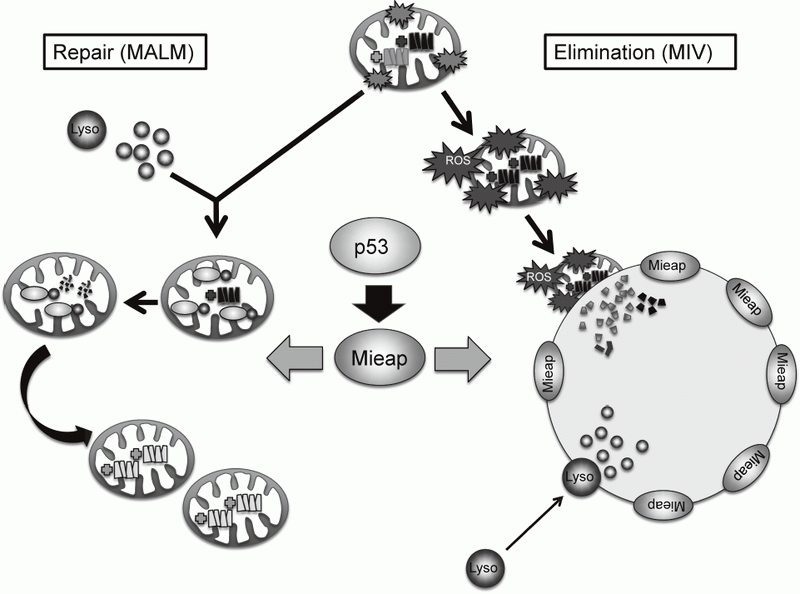
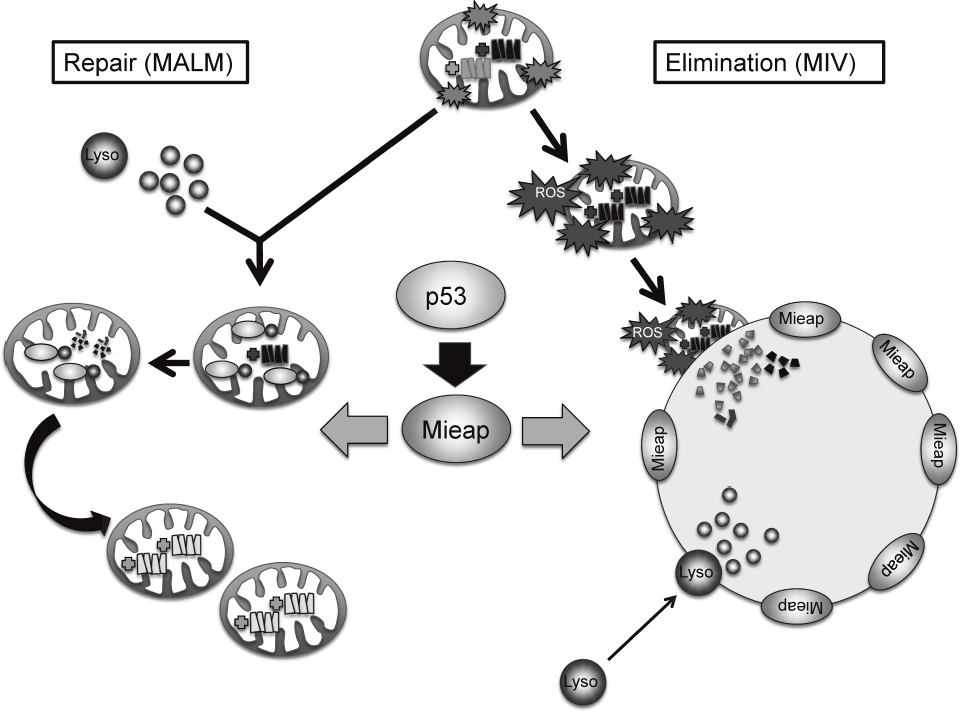
Mieap controls mitochondrial quality via two distinct novel mechanisms. One of the mechanisms has been designated MALM for Mieap-
induced accumulation of lysosome-like organelles
within mitochondria (PLoS ONE 6: e16054, 2011). In this mechanism, Mieap induces the accumulation of intramitochondrial lysosomal proteins in order to eliminate oxidized mitochondrial proteins in response to mitochondrial damage. This leads to a decrease in reactive oxygen species generation and an increase in mitochondrial ATP synthesis activity, implying MALM plays a role in repairing unhealthy mitochondria.
BNIP3 and NIX, mitochondrial outer membrane proteins, two Mieap-interacting proteins mediate the translocation of lysosomal proteins from cytosol into mitochondria during MALM by forming an unknown pore in the mitochondrial double membrane (PLoS ONE 7: e30767, 2012). 14-3-3g mediates the degradation of oxidized mitochondrial proteins within mitochondria during MALM (Scientific Reports 2: 379, 2012).
Alternatively, the other mechanism has been designated MIV for Mieap-induced vacuole (PLoS ONE 6: e16060, 2011). When MALM is inhibited, Mieap induces a vacuole-like structure, MIV. The MIV engulfs the damaged mitochondria and accumulates lysosomes, leading to the degradation of unhealthy mitochondria. MIV likely represents a novel mechanism for mitochondrial autophagy, also called "mitophagy". Therefore, Mieap controls mitochondrial quality by repairing or eliminating unhealthy mitochondria via MALM or MIV generation, respectively (Figure 1).
2. Frequent inactivation of Mieap-regulated mitochondrial quality control in human cancer
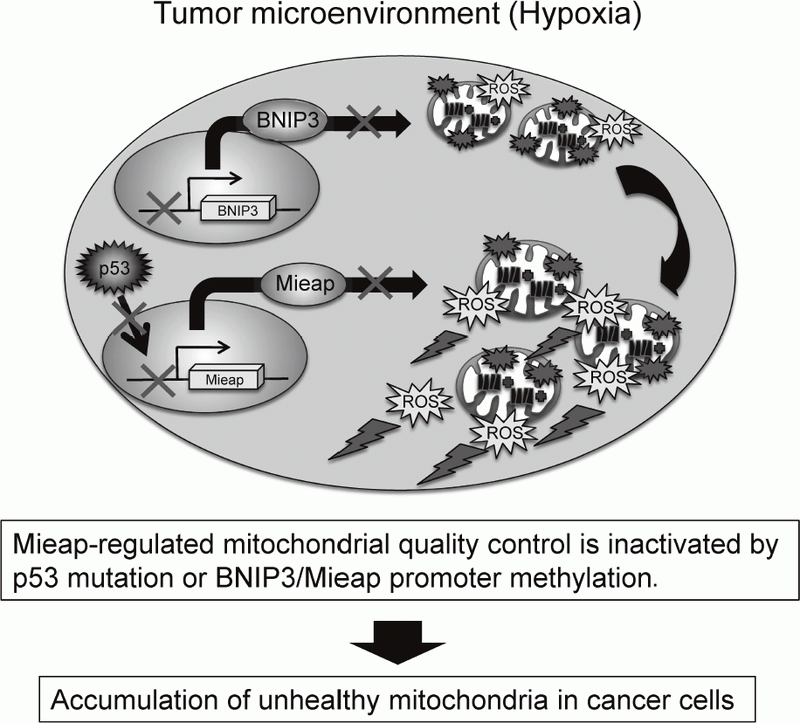
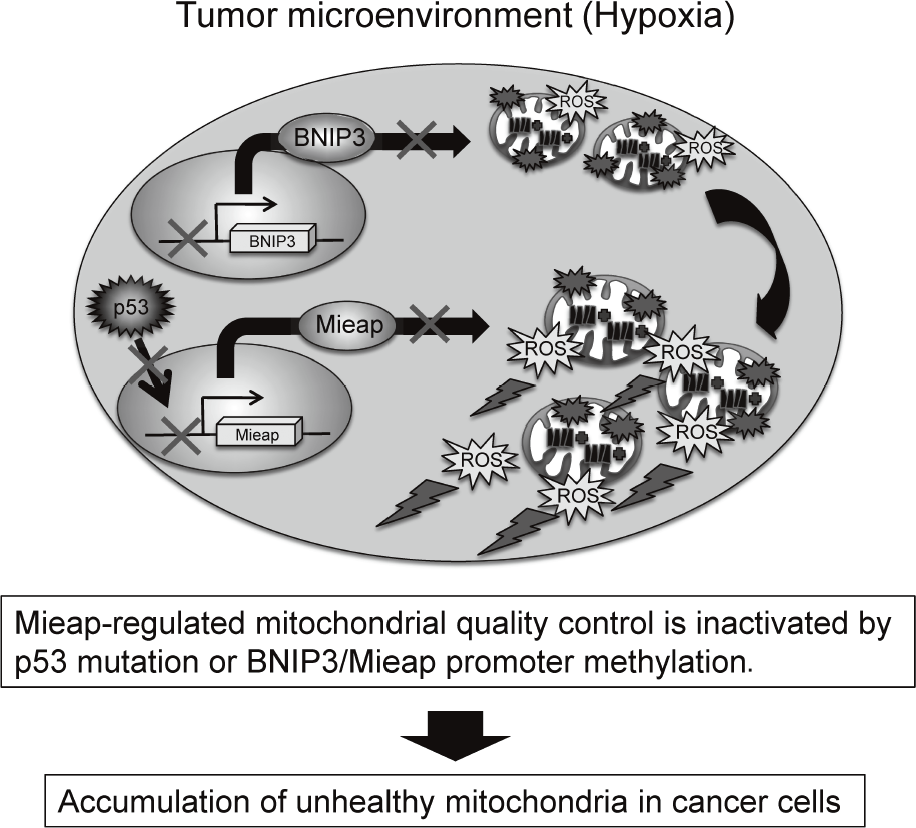
The accumulation of unhealthy mitochondria results in mitochondrial dysfunction, which has been implicated in aging, degenerative diseases and cancer. The Mieap-regulated mitochondrial quality control (MQC) was found to be frequently inactivated by p53 mutations or Mieap/BNIP3 promoter methylation in more than 70% of primary cancer tissues of colorectal cancer patients, leading to accumulation of unhealthy mitochondria and high levels of mitochondria reactive oxygen species (ROS) generation (Oncogenesis 5: e181, 2016).
The elevated mitochondrial ROS causes oxidative damage to DNA, RNA, protein, lipid, and so on. This induces genomic instability. The mitochondrial ROS contribute to tumor growth,
epithelial-to-mesenchymal transition, cancer inva-
sion, cancer metastasis, and tumor angiogenesis through the activation of HIF-1, NF-kB, MMPs, AKT, Erk1/2, JNK, and so on. Therefore, the Mieap-regulated MQC is a tumor suppressor for colorectal cancer (Figure 2).
3. Mieap-deficient cancer animal model
To clarify the in vivo role of the Mieap-
regulated MQC in tumorignenesis, the Mieap knockout mice were generated in our division. Using the Mieap knockout mice, the Mieap-
deficient ApcMin/+ mice were also generated and analyzed in order to elucidate the role of Mieap in colorectal cancer tumorigenesis (Scientific Reports 5: 12472, 2015).
Interestingly, the Mieap-deficient ApcMin/+ mice exhibited remarkably reduced lifespans compared with those of ApcMin/+ mice. Furthermore, a substantial increase in the number and size of intestinal polyps was found in the Mieap-deficient ApcMin/+ mice. Histopathologically, intestinal tumors in the Mieap-deficient ApcMin/+ mice clearly exhibited advanced grades of adenoma and adenocarcinomas. Unhealthy mitochondria dramatically accumulated in the tumor cells and generated high levels of ROS in the Mieap-deficient ApcMin/+ mice. These results suggest that the Mieap-regulated MQC pathway has a critical role in the suppression of intestinal tumor in vivo.
4. Non-canonical mitophagy induced by Mieap and its role in tumor suppression via cell death
Parkin/Pink1-mediated mitophagy plays a critical role in MQC, in which the damaged mitochondria are sequestered by autophagosomes, and degraded by fusion between autophagosomes and lysosomes. We found that the Mieap-
mediated non-canonical mitophagy as a new function of tumor suppressor p53. Mieap induces large vacuoles in cancer cells. The Mieap-induced vacuoles are generated from the mitochondria, and directly eat and degrade the cancer mitochondria. UVRAG regulates the Mieap-mediated non-canonical mitophagy. The Mieap-mediated
non-canonical mitophagy induces cell death via the iron-dependent production of reactive oxygen species (ROS). The Mieap-mediated non-
canonical mitophagy plays a critical role in tumor suppression via iron-dependent cell death.
Future prospects
Analyses of cancer cell lines, clinical cancer tissues, and cancer-mouse models enable us to understand the actual role of the Mieap-regulated MQC in human cancer initiation, progression, invasion, and metastasis. Finally, we will be able to establish a solid foundation for the development of new strategies for cancer prevention, diagnosis, and therapy in the future.
List of papers published in January 2017 - March 2018
Journal
1. Tsuneki M, Kinjo T, Mori T, Yoshida A, Kuyama K, Ohira A, Miyagi T, Takahashi K, Kawai A, Chuman H, Yamazaki N, Masuzawa M, Arakawa H. Survivin: A novel marker and potential therapeutic target for human angiosarcoma. Cancer Sci, 108:2295-2305, 2017
2. Nakamura Y, Arakawa H. Discovery of Mieap-regulated mitochondrial quality control as a new function of tumor suppressor p53. Cancer Sci, 108:809-817, 2017
