Annual Report 2018
Department of Thoracic Oncology
Koichi Goto, Seiji Niho, Kiyotaka Yoh, Shingo Matsumoto, Shigeki Umemura, Keisuke Kirita, Hibiki Udagawa, Yoshitaka Zenke, Takaya Ikeda, Takahiro Ohta, Tomoyuki Naito, Tetsuya Sakai, Akira Sugimoto, Ryo Itotani
Introduction
The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. The division aims to provide the highest quality treatment and establish new effective treatments against lung cancer and other thoracic malignancies through innovative clinical and translational research. To provide assistance to our patients through multidisciplinary care, the staff members of the division work closely with thoracic surgeons, radiation oncologists, pathologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.
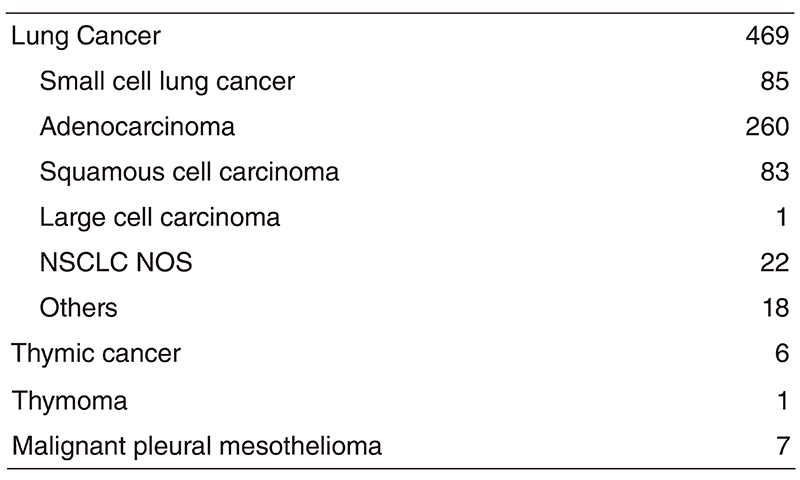
Table 1. Number of patients in 2018


Routine activities
Our Outpatient Clinic, managed by the staff members and senior residents, is open from Mondays to Fridays for the examination of all new referred patients and the evaluation of returning patients. Returning patients also receive oral chemotherapy and/or intravenous chemotherapy in the Ambulatory Care Center. Bronchoscopy with EBUS for diagnosis is performed from Monday to Thursday afternoons. Fluoroscopic-CT guided needle lung biopsies is carried out on Tuesday afternoons. For patient management, we use approximately 65 beds in mainly 8F, 6A, 6B and 5A wards.
Case conferences on thoracic surgery and medical oncology are scheduled on Tuesday evenings and Wednesday evenings, respectively. The staff members and residents of the division participate in a journal club on Monday and Wednesday mornings. At monthly meetings with physicians in private practice, the staff members and residents are teaching methods of reading for chest X-ray and CT scan films.
Research activities
Our research activities are focused on three areas:
1) development of new and effective diagnosis and treatment modalities in lung cancer;
2) collaborative studies with the Research Center for Innovative Oncology in the following areas: detection of driver mutation for small cell lung cancer; development of new diagnostic method of rare driver genomic alteration for lung cancer; correlation between genomic abnormalities and clinical characteristics and treatment in lung cancer; correlation between pathological features and sensitivity of treatments in lung cancer; and
3) translational research from bench to bed-side or from bed-side to bench for the development of innovative treatment strategies in lung cancer.
Especially, development of target sequence diagnosis methods by next generation sequencing for rare driver genomic alterations of lung cancer such as RET, ROS1, BRAF, MET and HER2 are currently under investigation collaborating with the Research Center for Innovative Oncology.
Clinical trials
The Department of Thoracic Oncology is currently conducting and participating in multi-institutional clinical studies for advanced lung cancer disease, such as the Japan Clinical Oncology Group (JCOG) trials, West Japan Oncology Group (WJOG) trials, Thoracic Oncology Research Group (TORG) trials, investigator initiated trials, and pharmaceutical company initiated global trials.
A nationwide genomic screening platform established by our department, whose name is LC-SCRUM-Japan, was initiated from 2013 and is now ongoing. As of March 13, 2019, 190 Japanese institutions participated in LC-SCRUM-Japan and 7,391 patients were enrolled. In addition, Chang Gung Memorial Hospital and 4 related hospitals in Taiwan participated in our genomic screening from March 2019, and LC-SCRUM-Japan became an Asian international genomic screening platform, whose name was changed to LCSCRUM-Asia. LC-SCRUM-Asia will support the development of novel therapeutic and diagnostic products in Asia and contribute to establish precision medicine in Asian countries. Many lung cancers with rare driver oncogenes, such as RET, ROS1 BRAF, MET, HER2 and KRAS genomic alterations were identified in our screening and they were entered into various clinical trials of molecular targeting agents. Based on the results of clinical trials targeting ROS1 fusion and BRAF mutation, crizotinib was approved for ROS1 fusion positive lung cancer in May 2017 and dabrafenib/trametinib was also approved for BRAF V600E positive lung cancer in March 2018. While RT-PCR kit, which was adapted in LCSCRUM-Japan screening for ROS1 fusion, was simultaneously approved using our screening data as a companion diagnostic for ROS1 positive lung cancer in January 2017. In the same way, a next generation sequencing (NGS) panel was first approved as a companion diagnostic for BARF mutation positive lung cancer in April 2018. Through the genomic screening, LC-SCRUMAsia should play a key role to establish precision medicine in lung cancer in Japan and Asian countries.
To establish some useful biomarkers to predict sensitivity of immune checkpoint inhibitors approved in lung cancer, such as nivolumab, pembrolizumab atezolizumab and durvalumab, LC-SCRUM-IBIS was initiated from February 2017 and enrollment of 1,017 patients was completed by May 2018. In this biomarker study, immunohistochemical (IHC) staining with four types of clones and whole exome sequencing were adapted. To confirm the effectiveness of NGS panel using plasma cell-free DNA (liquid biopsy system), we also started LC-SCRUMLiquid from December 2017 targeting 2,000 advanced lung cancers, and it is now ongoing. We will start umbrella-type clinical studies for lung cancer based on the liquid genomic screening from 2019.
To select optimal treatment for the individual patients with advanced lung cancer, we currently need to analyze many biomarkers including EGFR ALK, ROS1 and BRAF genomic alterations by NGS screening and PD-L1 expression by IHC using tissue samples. Since we would like to get as large amount of tissue samples as possible by bronchoscopy, we conduct a feasibility study of trans-bronchial cryobiopsy to evaluate its utility.
List of papers published in 2018
Journal
1. Yoh K, Takamochi K, Shukuya T, Hishida T, Tsuboi M, Sakurai H, Goto Y, Yoshida K, Ohde Y, Okumura S, Ohashi Y, Kunitoh H. Pattern of care in adjuvant therapy for resected Stage I non-small cell lung cancer: real-world data from Japan. Jpn J Clin Oncol, 49:63-68, 2019
2. Mizugaki H, Hamada A, Shibata T, Hosoda F, Nakamura H, Okuma Y, Shukuya T, Umemura S, Horiike A, Fukui T, Kogure Y, Daga H, Urata Y, Yamada K, Saeki S, Fujisaka Y, Nakamura Y, Sato M, Yoshida T, Hotta T, Oizumi S, Fujiwara Y, Ohe Y, Fujiwara Y. Exploration of germline variants responsible for adverse events of crizotinib in anaplastic lymphoma kinase-positive non-small cell lung cancer by target-gene panel sequencing. Lung Cancer, 128:20-25, 2019
3. Nakamura M, Kageyama SI, Niho S, Okumura M, Hojo H, Motegi A, Nakamura N, Zenda S, Yoh K, Goto K, Akimoto T. Impact of EGFR Mutation and ALK Translocation on Recurrence Pattern After Definitive Chemoradiotherapy for Inoperable Stage III Non-squamous Non-small-cell Lung Cancer. Clin Lung Cancer, 20:e256-e264, 2019
4. Tsuji D, Suzuki K, Kawasaki Y, Goto K, Matsui R, Seki N, Hashimoto H, Hama T, Yamanaka T, Yamamoto N, Itoh K. Risk factors associated with chemotherapy-induced nausea and vomiting in the triplet antiemetic regimen including palonosetron or granisetron for cisplatin-based chemotherapy: analysis of a randomized, double-blind controlled trial. Support Care Cancer, 27:1139-1147, 2019
5. Sugawara S, Nakagawa K, Yamamoto N, Nokihara H, Ohe Y, Nishio M, Takahashi T, Goto K, Maemondo M, Ichinose Y, Seto T, Sakai H, Gemma A, Imamura F, Shingyoji M, Saka H, Inoue A, Takeda K, Okamoto I, Kiura K, Morita S, Tamura T. Japanese subgroup analysis of a phase III study of S-1 versus docetaxel in non-small cell lung cancer patients after platinum-based treatment: EAST-LC. Int J Clin Oncol, 24:485-493, 2019
6. Watanabe S, Yoshioka H, Sakai H, Hotta K, Takenoyama M, Yamada K, Sugawara S, Takiguchi Y, Hosomi Y, Tomii K, Niho S, Yamamoto N, Nishio M, Ohe Y, Kato T, Takahashi T, Kamada A, Suzukawa K, Omori Y, Enatsu S, Nakagawa K, Tamura T. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line treatment for stage IV squamous nonsmall cell lung cancer: A phase 1b and randomized, open-label, multicenter, phase 2 trial in Japan. Lung Cancer, 129:55-62, 2019
7. Niho S, Hosomi Y, Okamoto H, Nihei K, Tanaka H, Hida T, Umemura S, Goto K, Akimoto T, Ohe Y. Carboplatin, S-1 and concurrent thoracic radiotherapy for elderly patients with locally advanced non-small cell lung cancer: a multicenter Phase I/II study. Jpn J Clin Oncol, 2019
8. Doi T, Yoh K, Shitara K, Takahashi H, Ueno M, Kobayashi S, Morimoto M, Okusaka T, Ueno H, Morizane C, Okano N, Nagashima F, Furuse J. First-in-human phase 1 study of novel dUTPase inhibitor TAS-114 in combination with S-1 in Japanese patients with advanced solid tumors. Invest New Drugs, 37:507- 518, 2019
9. Sekihara K, Aokage K, Oki T, Omori T, Katsumata S, Ueda T, Miyoshi T, Goto M, Nakasone S, Ichikawa T, Hishida T, Yoshida J, Hisakane K, Goto K, Tsuboi M. Long-term survival after complete resection of non-small-cell lung cancer in patients with interstitial lung disease. Interact Cardiovasc Thorac Surg, 26:638-643, 2018
10. Nakasone S, Mimaki S, Ichikawa T, Aokage K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Tsuboi M, Goto K, Tsuchihara K, Ishii G. Podoplanin-positive cancer-associated fibroblast recruitment within cancer stroma is associated with a higher number of single nucleotide variants in cancer cells in lung adenocarcinoma. J Cancer Res Clin Oncol, 144:893-900, 2018
11. Makinoshima H, Umemura S, Suzuki A, Nakanishi H, Maruyama A, Udagawa H, Mimaki S, Matsumoto S, Niho S, Ishii G, Tsuboi M, Ochiai A, Esumi H, Sasaki T, Goto K, Tsuchihara K. Metabolic Determinants of Sensitivity to Phosphatidylinositol 3-Kinase Pathway Inhibitor in Small-Cell Lung Carcinoma. Cancer Res, 78:2179-2190, 2018
12. Yamazaki S, Higuchi Y, Ishibashi M, Hashimoto H, Yasunaga M, Matsumura Y, Tsuchihara K, Tsuboi M, Goto K, Ochiai A, Ishii G. Collagen type I induces EGFR-TKI resistance in EGFR-mutated cancer cells by mTOR activation through Akt-independent pathway. Cancer Sci, 109:2063-2073, 2018
13. Sakai T, Tane K, Usui Y, Miyoshi T, Matsumoto S, Aokage K, Goto K, Suzuki M, Ishii G, Tsuboi M. Utility of Site-Specific Biopsy for Diagnosis of Desmoplastic Malignant Mesothelioma. Ann Thorac Surg, 106:e125-e128, 2018
14. Nakamura M, Onozawa M, Motegi A, Hojo H, Zenda S, Nakamura N, Udagawa H, Kirita K, Matsumoto S, Umemura S, Yoh K, Niho S, Goto K, Akimoto T. Impact of prophylactic cranial irradiation on pattern of brain metastases as a first recurrence site for limited-disease small-cell lung cancer. J Radiat Res, 59:767-773, 2018
15. Kiura K, Yoh K, Katakami N, Nogami N, Kasahara K, Takahashi T, Okamoto I, Cantarini M, Hodge R, Uchida H. Osimertinib in patients with epidermal growth factor receptor T790M advanced non-small cell lung cancer selected using cytology samples. Cancer Sci, 109:1177-1184, 2018
16. Hosomi Y, Tanai C, Yoh K, Goto Y, Sakai H, Kato T, Kaburagi T, Nishio M, Kim YH, Inoue A, Hasegawa Y, Isobe H, Tomizawa Y, Mori Y, Minato K, Yamada K, Ohashi Y, Kunitoh H. Characteristics and outcomes of patients with EGFR-mutation positive non small-cell lung cancer receiving gefitinib beyond radiological progression. Expert Opin Pharmacother, 19:1049-1056, 2018
17. Shimizu H, Suzuki K, Uchikura T, Tsuji D, Yamanaka T, Hashimoto H, Goto K, Matsui R, Seki N, Shimada T, Ikeda S, Ikegami N, Hama T, Yamamoto N, Sasaki T. Economic analysis of palonosetron versus granisetron in the standard triplet regimen for preventing chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy in Japan (TRIPLE phase III trial). J Pharm Health Care Sci, 4:31, 2018
18. Naito Y, Takahashi H, Shitara K, Okamoto W, Bando H, Kuwata T, Kuboki Y, Matsumoto S, Miki I, Yamanaka T, Watanabe A, Kojima M. Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study. Jpn J Clin Oncol, 48:559-564, 2018
19. Sunami K, Takahashi H, Tsuchihara K, Takeda M, Suzuki T, Naito Y, Sakai K, Dosaka-Akita H, Ishioka C, Kodera Y, Muto M, Wakai T, Yamazaki K, Yasui W, Bando H, Fujimoto Y, Fukuoka S, Harano K, Kawazoe A, Kimura G, Koganemaru S, Kogawa T, Kotani D, Kuboki Y, Matsumoto H, Matsumoto S, Mishima S, Nakamura Y, Sawada K, Shingaki S, Shitara K, Umemoto K, Umemura S, Yasuda K, Yoshino T, Yamamoto N, Nishio K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (Edition 10). Cancer Sci, 109:2980-2985, 2018
20. Iwama E, Sakai K, Azuma K, Harada D, Nosaki K, Hotta K, Nishio M, Kurata T, Fukuhara T, Akamatsu H, Goto K, Shimose T, Kishimoto J, Nakanishi Y, Nishio K, Okamoto I. Exploration of resistance mechanisms for epidermal growth factor receptor-tyrosine kinase inhibitors based on plasma analysis by digital polymerase chain reaction and next-generation sequencing. Cancer Sci, 109:3921-3933, 2018
21. Ochi N, Kawahara T, Nagasaki Y, Nakagawa N, Yamagishi T, Umemura S, Honda Y, Nakanishi H, Yamane H, Takigawa N. Publication of lung cancer clinical trials in the Japanese Clinical Trial Registry. Jpn J Clin Oncol, 48:995-1000, 2018
22. Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Lee M, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Wu YL. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol, 36:2244-2250, 2018
23. Kato Y, Ninomiya K, Ohashi K, Tomida S, Makimoto G, Watanabe H, Kudo K, Matsumoto S, Umemura S, Goto K, Ichihara E, Ninomiya T, Kubo T, Sato A, Hotta K, Tabata M, Toyooka S, Maeda Y, Kiura K. Combined effect of cabozantinib and gefitinib in crizotinib-resistant lung tumors harboring ROS1 fusions. Cancer Sci, 109:3149-3158, 2018
24. Niho S, Ikeda N, Michimae H, Suzuki K, Sakai H, Kaburagi T, Yoshiya K, Minato K, Kato T, Okamoto H, Seto T, Hosomi Y, Shimizu K, Saito H, Tsuchida M, Kunitoh H, Tsuboi M, Takeuchi M, Watanabe K. Overall Survival Results of the Feasibility Study of Adjuvant Chemotherapy With Docetaxel Plus Cisplatin Followed by Long-term Single-agent Administration of S-1 in Patients With Completely Resected Non-Small Cell Lung Cancer: Thoracic Oncology Research Group (TORG) 0809. Am J Clin Oncol, 2018
25. Kiura K, Imamura F, Kagamu H, Matsumoto S, Hida T, Nakagawa K, Satouchi M, Okamoto I, Takenoyama M, Fujisaka Y, Kurata T, Ito M, Tokushige K, Hatano B, Nishio M. Phase 3 study of ceritinib vs chemotherapy in ALK-rearranged NSCLC patients previously treated with chemotherapy and crizotinib (ASCEND-5): Japanese subset. Jpn J Clin Oncol, 48:367-375, 2018
26. Yamamoto N, Kenmotsu H, Goto K, Takeda K, Kato T, Takeda M, Horinouchi H, Saito I, Sarashina A, Tanaka T, Morsli N, Nakagawa K. An open-label feasibility study of nintedanib combined with docetaxel in Japanese patients with locally advanced or metastatic lung adenocarcinoma after failure of first-line chemotherapy. Cancer Chemother Pharmacol, 82:685-694, 2018
27. Atagi S, Mizusawa J, Ishikura S, Takahashi T, Okamoto H, Tanaka H, Goto K, Nakagawa K, Harada M, Takeda Y, Nogami N, Fujita Y, Kasai T, Kishi K, Sawa T, Takeda K, Tomii K, Satouchi M, Seto T, Ohe Y. Chemoradiotherapy in Elderly Patients With NonSmall-Cell Lung Cancer: Long-Term Follow-Up of a Randomized Trial (JCOG0301). Clin Lung Cancer, 19:e619-e627, 2018
28. Hida T, Seto T, Horinouchi H, Maemondo M, Takeda M, Hotta K, Hirai F, Kim YH, Matsumoto S, Ito M, Ayukawa K, Tokushige K, Yonemura M, Mitsudomi T, Nishio M. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci, 109:2863-2872, 2018
29. Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, Yang JJ, Yamamoto N, Ahn MJ, Takahashi T, Yamanaka T, Kemner A, Roychowdhury D, Paolini J, Usari T, Wilner KD, Goto K. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol, 36:1405-1411, 2018
30. Udagawa H, Umemura S, Murakami I, Mimaki S, Makinoshima H, Ishii G, Miyoshi T, Kirita K, Matsumoto S, Yoh K, Niho S, Tsuchihara K, Goto K. Genetic profiling-based prognostic prediction of patients with advanced small-cell lung cancer in large scale analysis. Lung Cancer, 126:182-188, 2018
31. Sato Y, Matsuda S, Maruyama A, Nakayama J, Miyashita T, Udagawa H, Umemura S, Yanagihara K, Ochiai A, Tomita M, Soga T, Tsuchihara K, Makinoshima H. Metabolic Characterization of Antifolate Responsiveness and Non-responsiveness in Malignant Pleural Mesothelioma Cells. Front Pharmacol, 9:1129, 2018
32. Ueda T, Aokage K, Mimaki S, Tane K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Kusumoto M, Suzuki K, Tsuchihara K, Nishikawa H, Goto K, Tsuboi M, Ishii G. Characterization of the tumor immune-microenvironment of lung adenocarcinoma associated with usual interstitial pneumonia. Lung Cancer, 126:162-169, 2018
33. Kato M, Nakamura H, Nagai M, Kubo T, Elzawahry A, Totoki Y, Tanabe Y, Furukawa E, Miyamoto J, Sakamoto H, Matsumoto S, Sunami K, Arai Y, Suzuki Y, Yoshida T, Tsuchihara K, Tamura K, Yamamoto N, Ichikawa H, Kohno T, Shibata T. A computational tool to detect DNA alterations tailored to formalin-fixed paraffin-embedded samples in cancer clinical sequencing. Genome Med, 10:44, 2018
