Annual Report 2018
Department of Hepatobiliary and Pancreatic Oncology
Masafumi Ikeda, Shuichi Mitsunaga, Izumi Ohno, Hiroshi Imaoka, Yusuke Hashimoto, Mitsuhito Sasaki, Kazuo Watanabe, Kumiko Umemoto, Gen Kimura, Taro Shibuki, Yuko Suzuki, Motoyasu Kan
Introduction
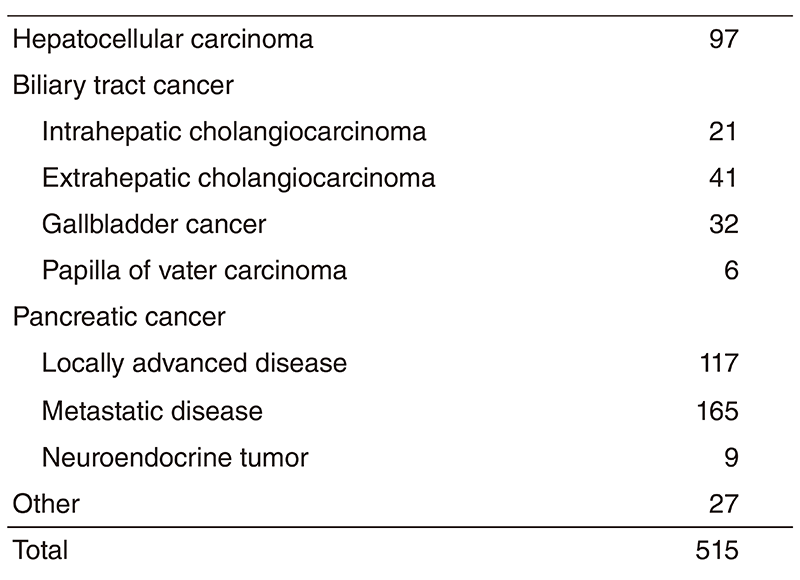
The Department of Hepatobiliary and Pancreatic Oncology is responsible for the diagnosis and treatment of patients with hepatic, biliary, and pancreatic cancers as well as interventional management by endoscopic or percutaneous procedures (Table 1). Our goal is to provide high-quality cancer treatment with adequate palliative care, and to develop novel and effective treatments and procedures through well-designed clinical trials and research.
Table 1. Number of cancer patients

The Team and What We Do
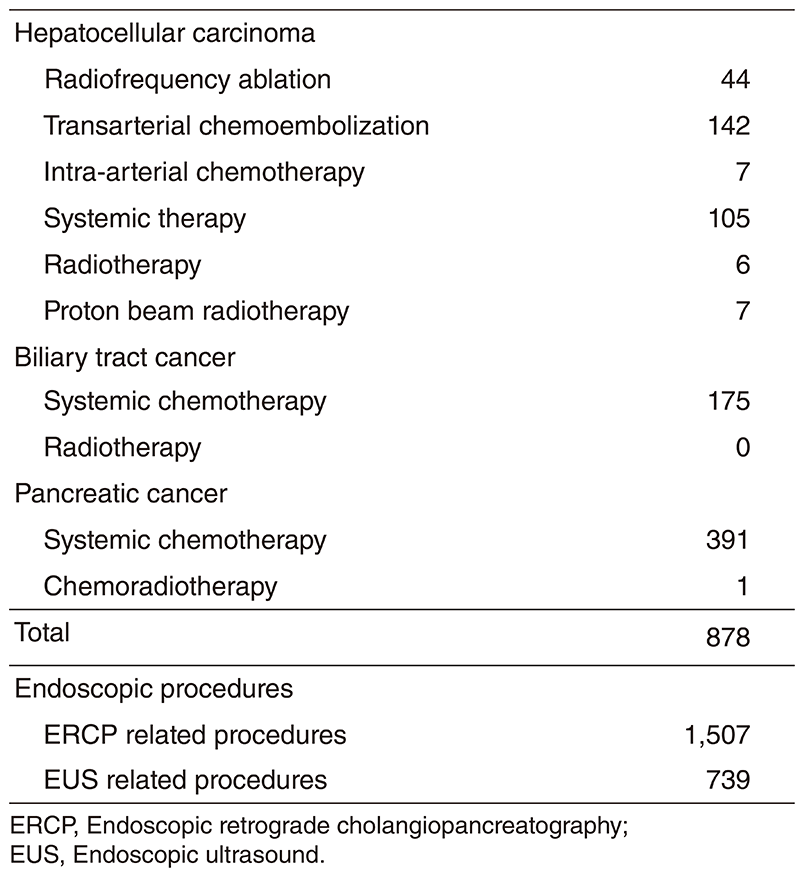
Our department is composed of six staff oncologists and six residents, with an average of 49 beds in the hospital. These doctors are divided into two teams and each team conducts clinical rounds for each admitted patient every morning and evening. The treatment strategies for individual patients are discussed in weekly tumor board conferences attended by medical oncologists, surgeons, radiologists, radiation oncologists, and pharmacists. Furthermore, we are also responsible for endoscopic abdominal ultrasonographic examinations, endoscopic or percutaneous ultrasound-guided biopsies of abdominal masses, local ablative therapy for liver tumors, endoscopic or percutaneous biliary drainage, abscess drainage and stenting for obstructive jaundice, etc (Table 2).
Research activities
1. Hepatocellular carcinoma (HCC)
Sorafenib and lenvatinib for first line and regorafenib and ramucirumab for second line are currently acknowledged as the standard Systemic therapy for advanced HCC. In clinical practice, various therapies are selected as first and second line treatments. Therefore, we are planning to conduct the prospective observational study to resolve the various clinical questions in unresectable HCC patients treated with systemic therapy in Japan. The aim of this study is to collect real-world data in Japan and to establish real-world evidence. Also, monotherapy of immune-checkpoint inhibitors, including nivolumab and pembrolizumab, showed the limited efficacy in patients with unresectable HCC, while the combination therapy of immunecheckpoint inhibitors plus VEGF inhibitors have some promising efficacy. To elucidate the efficacy and safety of these combinations, some clinical trials are under way.
2. Biliary tract cancer (BTC)
Gemcitabine plus cisplatin(GC) is established as one of the available standards of care for advanced BTC as first line treatment. Through retrospective analysis of 307 patients with advanced BTC who received GC therapy as the first-line chemotherapy at our institution, poor performance status, elevated serum lactate dehydrogenase, and elevated neutrophil-tolymphocyte ratio were revealed as independent unfavorable predictors. Immune-checkpoint inhibitors are promising agents for advanced BTC. At present, new developments in GC plus immune-checkpoint inhibitors are ongoing.
3. Pancreatic cancer (PC)
Gemcitabine (Gem) plus nab-paclitaxel and modified FOLFIRINOX has been established as a first line treatment of advanced PC, and both regimens are often used for advanced PC on daily practice. We conducted the retrospective comparison study to clarify which regimen was better as a first line chemotherapy, Gem plus nab-paclitaxel or modified FOLFIRINOX, and which regimen was better as a second line chemotherapy after the treatment of Gem plus nab-paclitaxel, modified FOLFIRINOX or S-1 alone.
4. Neuroendocrine neoplasms (NEN)
A number of drug classes have been shown to be potentially useful medical treatment agents for patients with unresectable pancreatic neuroendocrine tumors (PanNETs) including somatostatin analogues, molecular-targeted agents and cytotoxic anticancer agents. Since the optimal strategy for selection among these treatment options for patients with unresectable PanNETs has not yet been clarified, we propose a valuable MAP for optimal treatment selection for patients with unresectable PanNETs.
5. Endoscopic research
We investigated the usefulness of EUSguided n-butyl-2-cyanoacrylate injection therapy for ruptured isolated left gastric artery pseudoaneurysm and EUS-guided hepatogastrostomy and EUS-guided celiac neurolysis in advanced PC and BTC.
Clinical trials
Sixty-four clinical trials (sponsored: 36 trials, investigator-initiated: 28 trials) are ongoing, and 12 clinical trials (sponsored: eight trials, investigator-initiated: five trials) are being planned for the upcoming year.
1. HCC
A multicenter phase II trial of lenvatinib plus intra-arterial cisplatin and a randomized phase II trial comparing transarterial chemo embolization (TACE) using drug eluting Beads vs. conventional TACE (PRESIDENT) were ongoing. Some sponsored trials of lenvatinib plus pembrolizumab, bevacizumab plus atezolizumab, as the first line chemotherapy, and lenvatinib plus beta-catenin modulator, as the second line chemotherapy are ongoing.
2. BTC
An investigator-initiated trial of FOLFIRINOX and some sponsored trials of GC plus immune-checkpoint inhibitors as first line chemotherapy and resminostat plus S-1, capecitabine plus varlitinib, E7090, BAY1436032, JPH203, M7824, etc., for advanced BTCs refractory to Gem were conducted. For patients with occupational cholangiocarcinoma, a phase II trial of nivolumab was planned.
3. PC
Some clinical trials of a phase II/III trial of neoadjuvant S-1 and concurrent radiotherapy vs. Gem plus nab-paclitaxel for borderline resectable PC (GABARNANCE), Gem plus nab-paclitaxel vs. modified FOLFIRINOX vs. S-IROX for metastatic PC (JCOG1611), as the first line setting were ongoing. Some sponsored trials of HF10 plus Gem plus nab-paclitaxel, are ongoing as the first line setting. A phase I trial of tocilizumab plus Gem plus nab-paclitaxel was planned.
Education
For our residents, daily training is provided with group discussions on the daily practice of management of inpatients and outpatients. And they can learn the indications, administration and management of the adverse events from locoregional treatments to systemic chemotherapy for hepatic, biliary, and pancreatic cancer patients and the accompanying procedures to undertake diagnosis and interventional management, and so on. In addition, they can make presentations of their research in domestic and overseas meetings and make papers in English under the instruction of staff physicians.
Future prospects
The prognosis of patients with hepatic, biliary, and pancreatic cancers remains dismal, and standard treatments for theses cancer is limited. In Japan, the incidences of these cancers, especially HCC and BTC, are higher than those in Western countries. Therefore, we must conduct a lot of top-level novel and promising clinical trials and research. And it is necessary to develop biomarker research and endoscopic management alongside cancer treatment.
List of papers published in January 2017 - March 2018
Journal
1. Nakachi K, Konishi M, Ikeda M, Mizusawa J, Eba J, Okusaka T, Ishii H, Fukuda H, Furuse J. A randomized Phase III trial of adjuvant S-1 therapy vs observation alone in resected biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1202, ASCOT). Jpn J Clin Oncol, 48:392-395, 2018
2. Ioka T, Ueno M, Ueno H, Park JO, Chang HM, Sasahira N, Kanai M, Chung IJ, Ikeda M, Nakamori S, Mizuno N, Omuro Y, Yamaguchi T, Hara H, Sugimori K, Furuse J, Maguchi H, Furukawa M, Fukuzawa K, Kim JS, Yukisawa S, Takeuchi M, Okusaka T, Boku N, Hyodo I. TAS-118 (S-1 plus leucovorin) versus S-1 in patients with gemcitabine-refractory advanced pancreatic cancer: a randomised, open-label, phase 3 study (GRAPE trial). Eur J Cancer, 106:78-88, 2019
3. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 20:282-296, 2019
4. Naito T, Mitsunaga S, Miura S, Tatematsu N, Inano T, Mouri T, Tsuji T, Higashiguchi T, Inui A, Okayama T, Yamaguchi T, Morikawa A, Mori N, Takahashi T, Strasser F, Omae K, Mori K, Takayama K. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J Cachexia Sarcopenia Muscle, 10:73-83, 2019
5. Imaoka H, Sasaki M, Takahashi H, Hashimoto Y, Ohno I, Mitsunaga S, Watanabe K, Umemoto K, Kimura G, Suzuki Y, Kan M, Ikeda M. Alternate Endpoints for Phase II Trials in Advanced Neuroendocrine Tumors. Oncologist, 24:47-53, 2019
6. Ikeda M, Morimoto M, Tajimi M, Inoue K, Benhadji KA, Lahn MMF, Sakai D. A phase 1b study of transforming growth factor-beta receptor I inhibitor galunisertib in combination with sorafenib in Japanese patients with unresectable hepatocellular carcinoma. Invest New Drugs, 37:118-126, 2019
7. Ikeda M, Ohno I, Ueno H, Mitsunaga S, Hashimoto Y, Okusaka T, Kondo S, Sasaki M, Sakamoto Y, Takahashi H, Hara R, Kobayashi S, Nakamura O, Morizane C. Phase I study of resminostat, an HDAC inhibitor, combined with S-1 in patients with pre-treated biliary tract or pancreatic cancer. Invest New Drugs, 37:109-117, 2019
8. Suzuki Y, Kan M, Kimura G, Umemoto K, Watanabe K, Sasaki M, Takahashi H, Hashimoto Y, Imaoka H, Ohno I, Mitsunaga S, Ikeda M. Predictive factors of the treatment outcome in patients with advanced biliary tract cancer receiving gemcitabine plus cisplatin as first-line chemotherapy. J Gastroenterol, 54:281-290, 2019
9. Hashimoto Y, Ohno I, Takahashi H, Sasaki M, Imaoka H, Watanabe K, Umemoto K, Kimura G, Mitsunaga S, Ikeda M. EUS-guided n-butyl-2-cyanoacrylate injection therapy for ruptured isolated left gastric artery pseudoaneurysm. Endosc Ultrasound, 8:58-59, 2019
10. Nakachi K, Konishi M, Ikeda M, Shimada K, Okusaka T, Saiura A, Ishii H, Sugiyama M, Furuse J, Sakamoto H, Shimamura T, Ohta T. Feasibility study of postoperative adjuvant chemotherapy with S-1 in patients with biliary tract cancer. Int J Clin Oncol, 23:894- 899, 2018
11. Suzuki E, Kaneko S, Okusaka T, Ikeda M, Yamaguchi K, Sugimoto R, Aramaki T, Asagi A, Yasui K, Sano K, Hosokawa A, Kato N, Ishii H, Sato T, Furuse J. A multicenter Phase II study of sorafenib in Japanese patients with advanced hepatocellular carcinoma and Child Pugh A and B class. Jpn J Clin Oncol, 48:317- 321, 2018
12. Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K, Sata N, Miyashita K, Mizuno N, Tsuji K, Okusaka T, Furuse J. A phase II study of modified FOLFIRINOX for chemotherapy-naive patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol, 81:1017-1023, 2018
13. Sato Y, Ueno H, Ioka T, Ohkawa S, Ikeda M, Shimamura T, Tsuji A, Tsuchiya Y, Furuse J, Ishii H, Furuya K, Iguchi H, Saito Y, Kaniwa N, Sawada JI, Sakamoto H, Sekine A, Okusaka T, Yoshida T. SLCO1B1 Polymorphism Is a Drug Response Predictive Marker for Advanced Pancreatic Cancer Patients Treated With Gemcitabine, S-1, or Gemcitabine Plus S-1. Pancreas, 47:637-642, 2018
14. Shibuya H, Hijioka S, Sakamoto Y, Ito T, Ueda K, Komoto I, Kobayashi N, Kudo A, Yasuda H, Miyake H, Arita J, Kiritani S, Ikeda M, Imaoka H, Ueno M, Kobayashi S, Furuta M, Nagashio Y, Murohisa G, Aoki T, Matsumoto S, Motoya M, Azemoto N, Itakura J, Horiguchi S, Yogi T, Kawagoe T, Miyaoka Y, Imamura F, Senju M, Arioka H, Hara K, Imamura M, Okusaka T. Multi-center clinical evaluation of streptozocin-based chemotherapy for advanced pancreatic neuroendocrine tumors in Japan: focus on weekly regimens and monotherapy. Cancer Chemother Pharmacol, 82:661-668, 2018
15. Mizusawa J, Fukutomi A, Katayama H, Ishii H, Ioka T, Okusaka T, Ueno H, Ueno M, Ikeda M, Mizuno N, Ozaka M, Fukuda H, Furuse J. Protocol digest of randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel combination therapy for locally advanced pancreatic cancer: Japan clinical oncology group study (JCOG1407). Pancreatology, 18:841- 845, 2018
16. Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, Horiguchi S, Takahashi H, Suzuki E, Moriguchi M, Tsuji K, Otsuka T, Asagi A, Kojima Y, Takada R, Morizane C, Mizuno N, Ikeda M, Ueno M, Furuse J. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci, 109:2549-2557, 2018
17. Tak WY, Ryoo BY, Lim HY, Kim DY, Okusaka T, Ikeda M, Hidaka H, Yeon JE, Mizukoshi E, Morimoto M, Lee MA, Yasui K, Kawaguchi Y, Heo J, Morita S, Kim TY, Furuse J, Katayama K, Aramaki T, Hara R, Kimura T, Nakamura O, Kudo M. Phase I/II study of firstline combination therapy with sorafenib plus resminostat, an oral HDAC inhibitor, versus sorafenib monotherapy for advanced hepatocellular carcinoma in east Asian patients. Invest New Drugs, 36:1072-1084, 2018
18. Shiba S, Imaoka H, Shioji K, Suzuki E, Horiguchi S, Terashima T, Kojima Y, Okuno T, Sukawa Y, Tsuji K, Umemoto K, Asagi A, Todaka A, Ueno M, Ikeda M, Morizane C, Furuse J. Clinical characteristics of Japanese patients with epithelioid hemangioendothelioma: a multicenter retrospective study. BMC Cancer, 18:993, 2018
19. Okubo S, Mitsunaga S, Kato Y, Kojima M, Sugimoto M, Gotohda N, Takahashi S, Hayashi R, Konishi M. The prognostic impact of differentiation at the invasive front of biliary tract cancer. J Surg Oncol, 117:1278-1287, 2018
20. Takahashi D, Kojima M, Suzuki T, Sugimoto M, Kobayashi S, Takahashi S, Konishi M, Gotohda N, Ikeda M, Nakatsura T, Ochiai A, Nagino M. Profiling the Tumour Immune Microenvironment in Pancreatic Neuroendocrine Neoplasms with Multispectral Imaging Indicates Distinct Subpopulation Characteristics Concordant with WHO 2017 Classification. Sci Rep, 8:13166, 2018
21. Miura T, Mitsunaga S, Ikeda M, Ohno I, Takahashi H, Kuwata T, Ochiai A. Neural Invasion Spreads Macrophage-Related Allodynia via Neural Root in Pancreatic Cancer. Anesth Analg, 126:1729- 1738, 2018
22. Miura T, Mitsunaga S, Ikeda M, Ohno I, Takahashi H, Suzuki H, Irisawa A, Kuwata T, Ochiai A. Characterization of low active ghrelin ratio in patients with advanced pancreatic cancer. Support Care Cancer, 26:3811-3817, 2018
23. Fujioka R, Mochizuki N, Ikeda M, Sato A, Nomura S, Owada S, Yomoda S, Tsuchihara K, Kishino S, Esumi H. Change in plasma lactate concentration during arctigenin administration in a phase I clinical trial in patients with gemcitabine-refractory pancreatic cancer. PLoS ONE, 13:e0198219, 2018
24. Saito K, Ikeda M, Kojima Y, Hosoi H, Saito Y, Kondo S. Lipid profiling of pre-treatment plasma reveals biomarker candidates associated with response rates and hand-foot skin reactions in sorafenib-treated patients. Cancer Chemother Pharmacol, 82:677-684, 2018
25. Ikeda M, Kobayashi M, Tahara M, Kaneko S. Optimal management of patients with hepatocellular carcinoma treated with lenvatinib. Expert Opin Drug Saf, 17:1095-1105, 2018
26. Suzuki Y, Hashimoto Y, Shibuki T, Kan M, Kimura G, Umemoto K, Watanabe K, Sasaki M, Takahashi H, Imaoka H, Ohno I, Mitsunaga S, Ikeda M. Endoscopic Ultrasound-Guided Gallbladder Drainage for Aberrant Right Posterior Duct Obstruction Developing after Placement of a Covered Self-Expandable Metallic Stent in a Patient with Distal Biliary Obstruction. Case Rep Gastroenterol, 12:722-728, 2018
27. Morizane C, Ueno M, Ikeda M, Okusaka T, Ishii H, Furuse J. New developments in systemic therapy for advanced biliary tract cancer. Jpn J Clin Oncol, 48:703-711, 2018
