Annual Report 2018
Department of Pathology and Clinical Laboratories
Takeshi Kuwata, Masato Sugano, Akiko Nagatsuma, Sachiko Seo, Yuki Ohara, Yuki Nishimura, Shigeyuki Hasuo, Shigehisa Yoshida, Masahiro Karibe, Yuko Adegawa, Noriaki Sato, Masaki Takeda, Kumi Horiuchi, Seiji Iwasaki, Airi Uemura, Kenta Akie, Michiko Iida, Saki Sunohara, Masayuki Ito, Yuki Takahashi, Yasuharu Hashimoto, Yuichiro Yazaki, Michiteru Yamagishi, Keiko Arai, Mari Hisano, Yukihiro Okano, Mitsunori Tajima, Megumi Hasegawa, Mika Narikiyo, Yota Ikegami, Mika Sasanuma, Takaki Kobayashi, Aya Koike, Takuya Yamaguchi, Takuya Aiba, Keiko Nakai, Ayumi Setsuda, Ayumi Nakanishi, Ayumi Iwaya, Misato Nojiri, Tomoko Ikeda, Akira Miyaura, Minami Yokota, Risa Iseki, Ayaka Sasamoto, Yuka Yoshino, Masahiro Inoue, Izumi Suzuki, Sayuri Shibayama, Ikuko Takahashi, Kazuki Motohashi, Chihiro Kodama, Yukina Fukuda, Chiharu Eda, Sayuri Shibayama, Ayaka Takahashi, Midori Kawamura, Emiko Yoshikawa, Yuko Iwata, Megumi Yamaguchi, Noriko Sato, Mariko Kinoshita, Aiko Kimura, Yumiko Ito
Introduction
The Department of Pathology and Clinical Laboratories (DPCL) has two divisions: the Pathology Division and the Clinical Laboratory Division. Both divisions play a fundamental role in routine hospital service and support research activities at the NCCHE.
In 2012, the DPCL received ISO 15189: 2007 accreditation, ensuring quality control and quality assurance of testing including for clinical trials; it successfully transitioned to the newest version (ISO 15189: 2012) in 2014, and updated it in 2017.
The Team and What We Do
Pathology Division: All the pathologists engage in surgical pathology and research into cancer biology or development of new drugs. The number of samples examined at the department in 2018 is listed in Table 1.
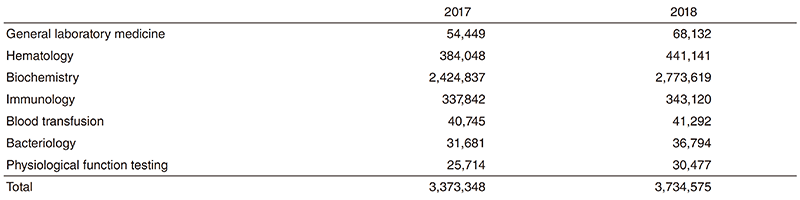
Clinical Laboratory Division: This division consists of eight sections; 1) general laboratory medicine, 2) hematology, 3) biochemistry and immunology, 4) physiological function testing, 5) bacteriology, 6) blood transfusion, 7) pathology and genetic testing, and 8) supporting laboratory testing in clinical studies. The numbers of tests performed in each division are listed in Table 2 and 3.
The total number of tests performed in the DPCL in 2018 increased about 11% compared with the previous year.
Since October 2018, we have converted all the testing performed in sections 1, 2 and 3 from outside laboratory control into inhouse management. Thus, we centralized our management into one organizational organ.
Table 1. Number of pathology and cytology samples examined at the Pathology Division in 2018
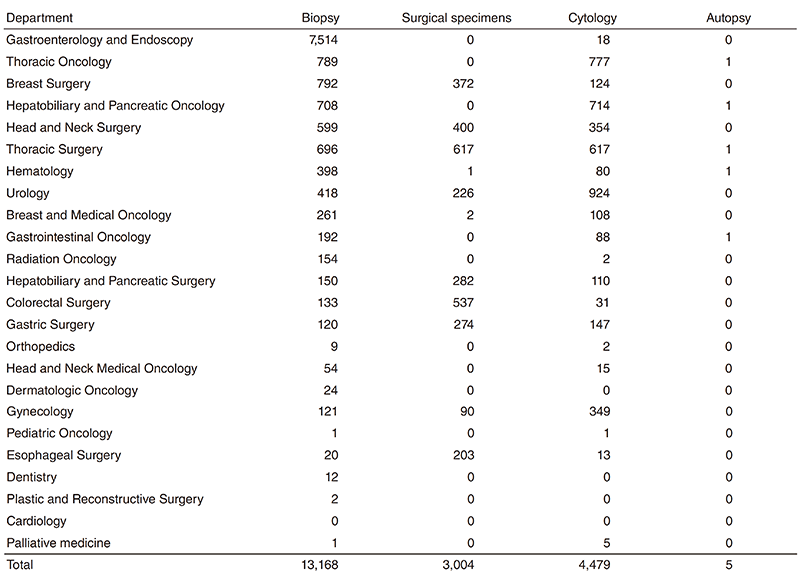

Table 2. Number of laboratory tests examined at the Clinical Laboratory Division in 2017 & 2018

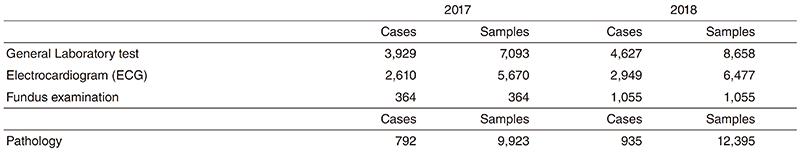
Research activities
All of the pathologists were involved in research activities at the Exploratory Oncology Research and Clinical Trial Center (EPOC). We strived to keep good quality formalin fixed paraffin embedded specimens and facilitated numerous research projects. Especially, we contributed to SCRUM-Japan (Cancer Genome Screening Project for Individualized Medicine in Japan) by using surgical specimens.
Clinical trials
The supporting laboratory testing in clinical studies, coordinated with pathology and physiological function testing, reinforces quality control and quality assurance for clinical testing performed in clinical trials at the NCCHE. Practically the Clinical Laboratory Division participated in all of the clinical trials undertaken at the NCCHE by providing laboratory data.
Education
The staff of the Clinical Laboratory Division regularly conduct study sessions or lecture meetings in order to improve their expertise and testing technique based on the requirements of ISO 15189.
Clinicopathological conferences are held regularly with each clinical department/ section. In the Pathology Division, conferencestyle training sessions are open weekly for the residents.
Future prospects
Pathological diagnosis and laboratory tests play a fundamental role not only in routine hospital work but also in medical research. Additionally, a genetic testing section has started to manage the next-generation sequencing-based gene panel testing to implement genomic cancer medicine from this summer. As an ISO15189- certified clinical laboratory, the DPCL will be continuously involved in investing in new diagnostic technologies, developing new drugs and conducting translational/clinical research in the NCCHE.
List of papers published in 2018
Journal
1. Miyashita T, Omori T, Nakamura H, Sugano M, Neri S, Fujii S, Hashimoto H, Tsuboi M, Ochiai A, Ishii G. Spatiotemporal characteristics of fibroblasts-dependent cancer cell invasion. J Cancer Res Clin Oncol, 145:373-381, 2019
2. Kawazoe A, Shitara K, Kuboki Y, Bando H, Kojima T, Yoshino T, Ohtsu A, Ochiai A, Togashi Y, Nishikawa H, Doi T, Kuwata T. Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein-Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer, 22:69- 76, 2019
3. Suzuki J, Kojima M, Aokage K, Sakai T, Nakamura H, Ohara Y, Tane K, Miyoshi T, Sugano M, Fujii S, Kuwata T, Ochiai A, Ito M, Suzuki K, Tsuboi M, Ishii G. Clinicopathological characteristics associated with necrosis in pulmonary metastases from colorectal cancer. Virchows Arch, 474:569-575, 2019
4. Oono Y, Kuwata T, Takashima K, Shinmura K, Hori K, Yoda Y, Ikematsu H, Shitara K, Kinoshita T, Yano T. Human epidermal growth factor receptor 2-, epidermal growth factor receptor-, and mesenchymal epithelial transition factor-positive sites of gastric cancer using surgical samples. Gastric Cancer, 22:335-343, 2019
5. Ichikawa T, Aokage K, Sugano M, Miyoshi T, Kojima M, Fujii S, Kuwata T, Ochiai A, Suzuki K, Tsuboi M, Ishii G. The ratio of cancer cells to stroma within the invasive area is a histologic prognostic parameter of lung adenocarcinoma. Lung Cancer, 118:30- 35, 2018
6. Nakasone S, Mimaki S, Ichikawa T, Aokage K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Tsuboi M, Goto K, Tsuchihara K, Ishii G. Podoplanin-positive cancer-associated fibroblast recruitment within cancer stroma is associated with a higher number of single nucleotide variants in cancer cells in lung adenocarcinoma. J Cancer Res Clin Oncol, 144:893-900, 2018
7. Katsumata S, Aokage K, Miyoshi T, Tane K, Nakamura H, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Hayashi R, Tsuboi M, Ishii G. Differences of tumor microenvironment between stage I lepidic-positive and lepidic-negative lung adenocarcinomas. J Thorac Cardiovasc Surg, 156:1679-1688.e2, 2018
8. Sakai T, Aokage K, Neri S, Nakamura H, Nomura S, Tane K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Iyoda A, Tsuboi M, Ishii G. Link between tumor-promoting fibrous microenvironment and an immunosuppressive microenvironment in stage I lung adenocarcinoma. Lung Cancer, 126:64-71, 2018
9. Mishima S, Kawazoe A, Matsumoto H, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Nonte EM, Chintharlapalli S, Nasir A, Kuwata T, Shitara K. Efficacy and safety of ramucirumab-containing chemotherapy in patients with pretreated metastatic gastric neuroendocrine carcinoma. ESMO open, 3:e000443, 2018
10. Naito Y, Takahashi H, Shitara K, Okamoto W, Bando H, Kuwata T, Kuboki Y, Matsumoto S, Miki I, Yamanaka T, Watanabe A, Kojima M. Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study. Jpn J Clin Oncol, 48:559-564, 2018
11. Tada Y, Togashi Y, Kotani D, Kuwata T, Sato E, Kawazoe A, Doi T, Wada H, Nishikawa H, Shitara K. Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8+ T cells in the tumor microenvironment. J Immunother Cancer, 6:106, 2018
12. Miura T, Mitsunaga S, Ikeda M, Ohno I, Takahashi H, Kuwata T, Ochiai A. Neural Invasion Spreads Macrophage-Related Allodynia via Neural Root in Pancreatic Cancer. Anesth Analg, 126:1729- 1738, 2018
13. Miura T, Mitsunaga S, Ikeda M, Ohno I, Takahashi H, Suzuki H, Irisawa A, Kuwata T, Ochiai A. Characterization of low active ghrelin ratio in patients with advanced pancreatic cancer. Support Care Cancer, 26:3811-3817, 2018
14. Oono Y, Kuwata T, Takashima K, Yoda Y, Ikematsu H, Shitara K, Kinoshita T, Yano T. Clinicopathological features and endoscopic findings of HER2-positive gastric cancer. Surg Endosc, 32:3964- 3971, 2018
15. Yanagihara K, Kubo T, Mihara K, Kuwata T, Ochiai A, Seyama T, Yokozaki H. Establishment of a novel cell line from a rare human duodenal poorly differentiated neuroendocrine carcinoma. Oncotarget, 9:36503-36514, 2018
16. Nakamura H, Ichikawa T, Nakasone S, Miyoshi T, Sugano M, Kojima M, Fujii S, Ochiai A, Kuwata T, Aokage K, Suzuki K, Tsuboi M, Ishii G. Abundant tumor promoting stromal cells in lung adenocarcinoma with hypoxic regions. Lung Cancer, 115:56-63, 2018
17. Nakajo K, Oono Y, Kuwata T, Yano T. A case of a protruded lesion formed by a poorly differentiated intramucosal adenocarcinoma of the stomach: an immunohistochemical analysis. Clin J Gastroenterol, 11:127-132, 2018
18. Nakajo K, Oono Y, Kuwata T. Case of pyloric gland adenoma accompanied by a component of foveolar epithelial-type adenoma within the lesion. Dig Endosc, 30:673, 2018
19. Ueda T, Aokage K, Mimaki S, Tane K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Kusumoto M, Suzuki K, Tsuchihara K, Nishikawa H, Goto K, Tsuboi M, Ishii G. Characterization of the tumor immune-microenvironment of lung adenocarcinoma associated with usual interstitial pneumonia. Lung Cancer, 126:162-169, 2018
20. Ueda T, Aokage K, Nishikawa H, Neri S, Nakamura H, Sugano M, Tane K, Miyoshi T, Kojima M, Fujii S, Kuwata T, Ochiai A, Kusumoto M, Suzuki K, Tsuboi M, Ishii G. Immunosuppressive tumor microenvironment of usual interstitial pneumonia-associated squamous cell carcinoma of the lung. J Cancer Res Clin Oncol, 144:835-844, 2018
