Annual Report 2018
Department of Experimental Therapeutics
Toshihiko Doi, Yasutoshi Kuboki, Kiyotaka Yoh, Yoichi Naito, Kohei Shitara, Takahiro Kogawa, Hideaki Takahashi, Kenichi Harano, Junichiro Yuda, Nobuhiko Yamauchi, Shigehiro Koganemaru
Introduction
The NCC-EPOC Phase I Group was organized to promote early drug development, especially the first human (FIH) trial and in 2012 and the phase I group comprised two sub-units (NCCE-Kashiwa & NCC-Tsukiji) organized by each hospital. The goal of both (or each of) the units was to perform an initial clinical evaluation of promising new anti-cancer compounds emerging from the laboratory. Our Phase 1 unit is the largest program in Japan - indeed in Asia - and we help develop new cancer drugs through early-phase trials.
In April 2013, the Department of Experimental Therapeutics was launched to strongly promote the EPOC missions as previously described. The members of the Department of Experimental Therapeutics comprised specialists in oncology fields. We also contributed to IIT using unapproved drugs and new academic seeds.
The Team and What We Do
Our team has conducted and managed early drug development, especially first-in-human (FIH) trials.
Research activities
This department is key to developing new anti-cancer drugs in our center as well as nationally and while conducting FIH trials is the top priority, we also perform phase-I trials. Recently, we joined the global phase-I trial to accelerate new drug development in Japan. Video or teleconferences have been held with EU and US sites and we are discussing details of patient enrollment as well as further developmental strategy. Routine web conferences are also held between Kashiwa and Tsukiji campuses every Friday morning and we share information about adverse events and patient enrollment as well as referring candidates to each other to accelerate enrollment. Several IIT-FIH using new class seeds are conducted by each unit as well as unapproved company agents
Clinical trials
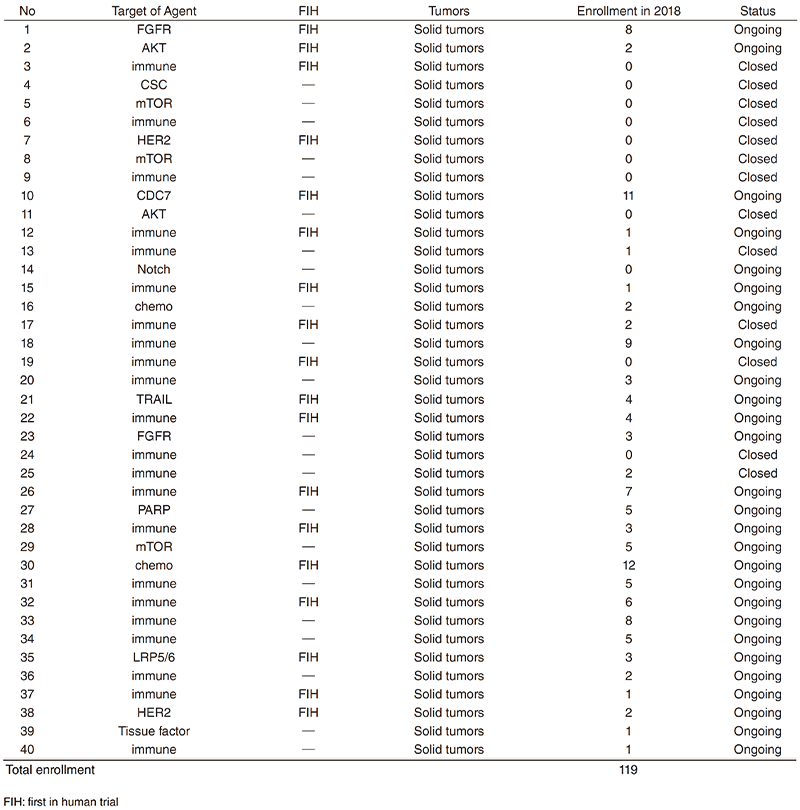
In 2018, 40 phase-I trials were conducted. (Table 1).
Table 1. Phase 1 Trials in 2018

List of papers published in 2018
Journal
1. Shimokawa M, Hayashi T, Kogawa T, Matsui R, Mizuno M, Kikkawa F, Saeki T, Aiba K, Tamura K. Evaluation of Combination Antiemetic Therapy on CINV in Patients With Gynecologic Cancer Receiving TC Chemotherapy. Anticancer Res, 39:225-230, 2019
2. Yoh K, Takamochi K, Shukuya T, Hishida T, Tsuboi M, Sakurai H, Goto Y, Yoshida K, Ohde Y, Okumura S, Ohashi Y, Kunitoh H. Pattern of care in adjuvant therapy for resected Stage I non-small cell lung cancer: real-world data from Japan. Jpn J Clin Oncol, 49:63-68, 2019
3. Nakamura M, Kageyama SI, Niho S, Okumura M, Hojo H, Motegi A, Nakamura N, Zenda S, Yoh K, Goto K, Akimoto T. Impact of EGFR Mutation and ALK Translocation on Recurrence Pattern After Definitive Chemoradiotherapy for Inoperable Stage III Non-squamous Non-small-cell Lung Cancer. Clin Lung Cancer, 20:e256-e264, 2019
4. Naito T, Umemura S, Nakamura H, Zenke Y, Udagawa H, Kirita K, Matsumoto S, Yoh K, Niho S, Motoi N, Aokage K, Tsuboi M, Ishii G, Goto K. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: A case report. Thoracic cancer, 10:1285-1288, 2019
5. Yuda J, Odawara J, Minami M, Muta T, Kohno K, Tanimoto K, Eto T, Shima T, Kikushige Y, Kato K, Takenaka K, Iwasaki H, Minami Y, Ohkawa Y, Akashi K, Miyamoto T. TKIs induce alternative spliced BCR-ABLIns35bp variant via inhibition of RNA polymeraseII on genomic BCR-ABL. Blood advances, 2019
6. Terao T, Yuda J, Yamauchi N, Miyamoto K, Minami M, Kojima M, Sugano M, Kuwata T, Minami Y. Classic hairy cell leukemia with bone marrow necrosis after cladribine. Jpn J Clin Hematol, 2019
7. Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer, 19:196, 2019
8. Kawazoe A, Shitara K, Kuboki Y, Bando H, Kojima T, Yoshino T, Ohtsu A, Ochiai A, Togashi Y, Nishikawa H, Doi T, Kuwata T. Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein-Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer, 22:69- 76, 2019
9. Yonemori K, Shimomura A, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hashimoto J, Yamamoto H, Hirakawa A, Michimae H, Hamada A, Yoshida T, Sukigara T, Tamura K, Fujiwara Y. A phase I/II trial of olaparib tablet in combination with eribulin in Japanese patients with advanced or metastatic triple-negative breast cancer previously treated with anthracyclines and taxanes. Eur J Cancer, 109:84-91, 2019
10. Mishima S, Kawazoe A, Nakamura Y, Sasaki A, Kotani D, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Kuwata T, Tsuji A, Shitara K. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer, 7:24, 2019
11. Doi T, Yang JC, Shitara K, Naito Y, Cheng AL, Sarashina A, Pronk LC, Takeuchi Y, Lin CC. Phase I Study of the Focal Adhesion Kinase Inhibitor BI 853520 in Japanese and Taiwanese Patients with Advanced or Metastatic Solid Tumors. Target Oncol, 14:57- 65, 2019
12. Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, Lopez J, Doi T, van Brummelen EMJ, Cristescu R, Yang P, Emancipator K, Stein K, Ayers M, Joe AK, Lunceford JK. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol, 37:318-327, 2019
13. Shimomura A, Yamamoto N, Kondo S, Fujiwara Y, Suzuki S, Yanagitani N, Horiike A, Kitazono S, Ohyanagi F, Doi T, Kuboki Y, Kawazoe A, Shitara K, Ohno I, Banerji U, Sundar R, Ohkubo S, Calleja EM, Nishio M. First-in-Human Phase I Study of an Oral HSP90 Inhibitor, TAS-116, in Patients with Advanced Solid Tumors. Mol Cancer Ther, 18:531-540, 2019
14. Sawaki A, Yamada Y, Yamaguchi K, Nishina T, Doi T, Satoh T, Chin K, Boku N, Omuro Y, Komatsu Y, Hamamoto Y, Koizumi W, Saji S, Shah MA, Van Cutsem E, Kang YK, Iwasaki J, Kuriki H, Ohtsuka W, Ohtsu A. Regional differences in advanced gastric cancer: exploratory analyses of the AVAGAST placebo arm. Gastric Cancer, 21:429-438, 2018
15. Matsubara N, Naito Y, Nakano K, Fujiwara Y, Ikezawa H, Yusa W, Namiki M, Okude T, Takahashi S. Lenvatinib in combination with everolimus in patients with advanced or metastatic renal cell carcinoma: A phase 1 study. Int J Urol, 25:922-928, 2018
16. Harano K, Wang Y, Lim B, Seitz RS, Morris SW, Bailey DB, Hout DR, Skelton RL, Ring BZ, Masuda H, Rao AUK, Laere SV, Bertucci F, Woodward WA, Reuben JM, Krishnamurthy S, Ueno NT. Rates of immune cell infiltration in patients with triple-negative breast cancer by molecular subtype. PLoS ONE, 13:e0204513, 2018
17. Kono M, Fujii T, Matsuda N, Harano K, Chen H, Wathoo C, Joon AY, Tripathy D, Meric-Bernstam F, Ueno NT. Somatic mutations, clinicopathologic characteristics, and survival in patients with untreated breast cancer with bone-only and non-bone sites of first metastasis. J Cancer, 9:3640-3646, 2018
18. Kogawa T, Fujii T, Fouad TM, Liu DD, Harano K, Masuda H, Iwase T, Barnett C, Park YS, Lim B, Tripathy D, Litton JK, Ueno NT. Impact of change in body mass index during neoadjuvant chemotherapy and survival among breast cancer subtypes. Breast Cancer Res Treat, 171:501-511, 2018
19. Ding Q, Wang Y, Zuo Z, Gong Y, Krishnamurthy S, Li CW, Lai YJ, Wei W, Wang J, Manyam GC, Diao L, Zhang X, Lin F, Symmans WF, Sun L, Liu CG, Liu X, Debeb BG, Ueno NT, Harano K, Alvarez RH, Wu Y, Cristofanilli M, Huo L. Decreased expression of microRNA-26b in locally advanced and inflammatory breast cancer. Hum Pathol, 77:121-129, 2018
20. Naito Y, Urasaki T. Precision medicine in breast cancer. Chin Clin Oncol, 7:29, 2018
21. Nakamura M, Onozawa M, Motegi A, Hojo H, Zenda S, Nakamura N, Udagawa H, Kirita K, Matsumoto S, Umemura S, Yoh K, Niho S, Goto K, Akimoto T. Impact of prophylactic cranial irradiation on pattern of brain metastases as a first recurrence site for limited-disease small-cell lung cancer. J Radiat Res, 59:767-773, 2018
22. Kiura K, Yoh K, Katakami N, Nogami N, Kasahara K, Takahashi T, Okamoto I, Cantarini M, Hodge R, Uchida H. Osimertinib in patients with epidermal growth factor receptor T790M advanced non-small cell lung cancer selected using cytology samples. Cancer Sci, 109:1177-1184, 2018
23. Hosomi Y, Tanai C, Yoh K, Goto Y, Sakai H, Kato T, Kaburagi T, Nishio M, Kim YH, Inoue A, Hasegawa Y, Isobe H, Tomizawa Y, Mori Y, Minato K, Yamada K, Ohashi Y, Kunitoh H. Characteristics and outcomes of patients with EGFR-mutation positive nonsmall-cell lung cancer receiving gefitinib beyond radiological progression. Expert Opin Pharmacother, 19:1049-1056, 2018
24. Matsumoto H, Sasaki A, Nakamura Y, Kawazoe A, Kuboki Y, Okinaka K, Shitara K. Tuberculous Meningitis during Chemotherapy for Advanced Gastric Cancer. Case reports in oncology, 11:228-233, 2018
25. Kuboki Y, Schatz CA, Koechert K, Schubert S, Feng J, Wittemer-Rump S, Ziegelbauer K, Krahn T, Nagatsuma AK, Ochiai A. In situ analysis of FGFR2 mRNA and comparison with FGFR2 gene copy number by dual-color in situ hybridization in a large cohort of gastric cancer patients. Gastric Cancer, 21:401-412, 2018
26. Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, Alsina M, Ghidini M, Faustino C, Gorbunova V, Zhavrid E, Nishikawa K, Hosokawa A, Yalcin S, Fujitani K, Beretta GD, Van Cutsem E, Winkler RE, Makris L, Ilson DH, Tabernero J. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 19:1437-1448, 2018
27. Mishima S, Kawazoe A, Matsumoto H, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Nonte EM, Chintharlapalli S, Nasir A, Kuwata T, Shitara K. Efficacy and safety of ramucirumab-containing chemotherapy in patients with pretreated metastatic gastric neuroendocrine carcinoma. ESMO open, 3:e000443, 2018
28. Segal NH, He AR, Doi T, Levy R, Bhatia S, Pishvaian MJ, Cesari R, Chen Y, Davis CB, Huang B, Thall AD, Gopal AK. Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/ CD137 Agonist, in Patients with Advanced Cancer. Clin Cancer Res, 24:1816-1823, 2018
29. Bang YJ, Takano T, Lin CC, Fasanmade A, Yang H, Danaee H, Asato T, Kalebic T, Wang H, Doi T. TAK-264 (MLN0264) in Previously Treated Asian Patients with Advanced Gastrointestinal Carcinoma Expressing Guanylyl Cyclase C: Results from an Open-Label, Non-randomized Phase 1 Study. Cancer Res Treat, 50:398-404, 2018
30. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA oncology, 4:e180013, 2018
31. Nishina T, Takahashi S, Iwasawa R, Noguchi H, Aoki M, Doi T. Safety, pharmacokinetic, and pharmacodynamics of erdafitinib, a pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, in patients with advanced or refractory solid tumors. Invest New Drugs, 36:424-434, 2018
32. Naito Y, Takahashi H, Shitara K, Okamoto W, Bando H, Kuwata T, Kuboki Y, Matsumoto S, Miki I, Yamanaka T, Watanabe A, Kojima M. Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study. Jpn J Clin Oncol, 48:559-564, 2018
33. Sunami K, Takahashi H, Tsuchihara K, Takeda M, Suzuki T, Naito Y, Sakai K, Dosaka-Akita H, Ishioka C, Kodera Y, Muto M, Wakai T, Yamazaki K, Yasui W, Bando H, Fujimoto Y, Fukuoka S, Harano K, Kawazoe A, Kimura G, Koganemaru S, Kogawa T, Kotani D, Kuboki Y, Matsumoto H, Matsumoto S, Mishima S, Nakamura Y, Sawada K, Shingaki S, Shitara K, Umemoto K, Umemura S, Yasuda K, Yoshino T, Yamamoto N, Nishio K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (Edition 10). Cancer Sci, 109:2980-2985, 2018
34. Tada Y, Togashi Y, Kotani D, Kuwata T, Sato E, Kawazoe A, Doi T, Wada H, Nishikawa H, Shitara K. Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8+ T cells in the tumor microenvironment. J Immunother Cancer, 6:106, 2018
35. Udagawa H, Umemura S, Murakami I, Mimaki S, Makinoshima H, Ishii G, Miyoshi T, Kirita K, Matsumoto S, Yoh K, Niho S, Tsuchihara K, Goto K. Genetic profiling-based prognostic prediction of patients with advanced small-cell lung cancer in large scale analysis. Lung Cancer, 126:182-188, 2018
