Annual Report 2018
Clinical Research Support Office
Research Management Division:Akihiro Sato
Project Management / Regulatory Affairs Management Section:Nozomu Fuse
Clinical Trial Management SectionMiki Fukutani, Hiromi Ono, Sakiko Kuroda, Rena Sato, Hitomi Tamura, Masako Nakamoto, Makoto Fukui, Yuichi Mikamoto, Shinobu Iida, Mitiko Suzuki, Sachiko Sakamoto, Kozi Takahasi, Maiko Takakusa, Hikari Sima
Data Management SectionYuya Ikeda, Natuko Iwasaki, Ayako Sugama , Kyouko Tokita, Kaori Tobayama, Tukiko Higuchi, Nami Hirano, Seiko Matsuda, Akiomi Yoshida, Michio Ohono, Kei Ikeno, Kazuko Tsukamoto
Biostatistics SectionShogo Nomura, Masahi Wakabayasi
Pharmacovigilance SectionMasato Yonemura, Noriko Fuzisiro, Kanae Kurashige
Information Technology Management SectionYoshihiro Aoyagi
Translational Research Management DivisionKatsuya Tsuchihara
Biobank Translational Research Support SectionWataru Okamoto
Biobank Research ConciergeAkiko Nakayama
Translational Research SupportIzumi Miki, Yasutoshi Sakamoto
Research Sample Management SupportYasuko Tada
Seeds Development Support SectionKatsuji Aikawa
Clinical Research Coordinating DivisionTakayuki Yoshino, Masafumi Ikeda, Yoichi Naito, Kiyotaka Yoh, Takashi Kojima, Nobuaki Matsubara, Yasutoshi Kuboki, Takahiro Sakai, Yukie Kimura, Miyuki Hara, Yuko Ito, Ikumi Tamaki, Mayumi Shirase, Keiko Yamamoto, Chiyo Ito, Koko Komata, Aya Shinbu, Chiharu Hirano, Kiyoko Adachi, Keiko Abe, Rie Taniguchi, Kyoko Tsuyuki, Hiromi Motoyanagi, Kazumi Yamaguchi, Yasuko Yoshihara, Kyoko Uehara, Noriko Noda, Yoshimi Fujiki, Junko Tsukada, Megumi Hodota, Rikako Tanaka, Megumi Futamura, Masami Sasaki, Mizuho Ibaraki, Aki Hashimoto, Mikina Takiguchi, Tomoko Matsumura, Atsuko Katagiri, Haruna Ariyoshi, Hikaru Matsuki, Masumi Kudo, Yoshimi Izumi, Minako Suzuki, Mayumi Nagino, Fumiko Kase, Tomoko Watanabe, Azusa Funaba, Mizuho Ibaraki, Misato Makishi, Ayae Minobe, Natshuko Takagi, Fumiko Arai, Ayaka Ishikawa, Keiko Abe, Aya Iwamoto, Ayaka Sakamoto, Saori Yokoba, Sayuri Nochi, Mari Takahashi, Kazumi Yamaguchi, Yasuko Yosihara
Introduction
The Office of Clinical Research supports the clinical trial program conducted in the National Cancer Center Hospital East (NCCHE)
The Team and What We Do
Research Management Division:
Through the clinical datacenter, we support study management, site visit monitoring and gathering safety information and bio statistics.
Translational Research Management Division
We support translational research including seeds development, biomarker discovery research, and development of in vitro diagnostics and companion diagnostics.
The Clinical Research Coordinating Division mainly focuses on the first-in-human clinical trials as well as investigator-initiated innovative clinical trials for unmet medical needs.
Clinical trials
Research Management Division:
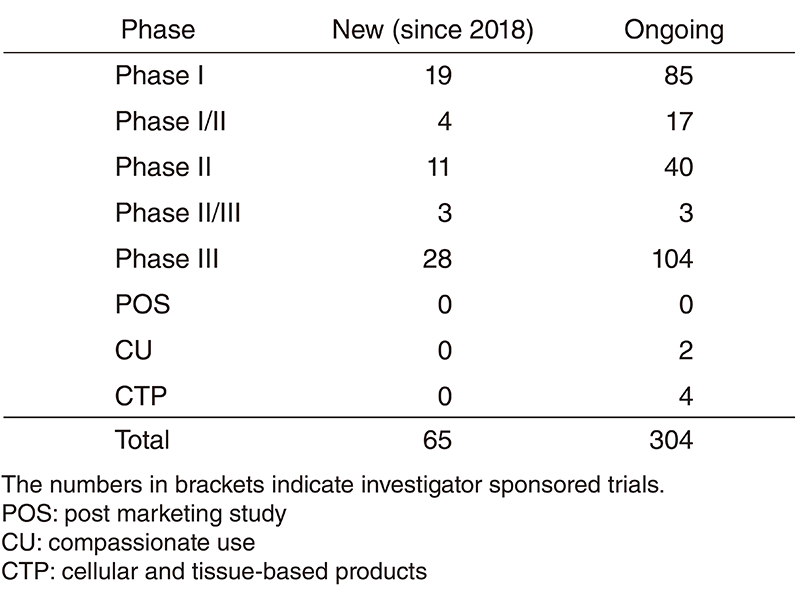
- In 2018, ten Investigational new drug (IND) trials started their enrollment.
Translational Research Management Division:
We have supported the Nationwide Genome Screening Project: SCRUM-Japan since 2015, which had collected clinical and genomic data on more than 10,000 patients by the end of 2018.
We supported ctDNA analysis (liquid biopsy) and international programs in 2018.
We started 77 sponsored initiated clinical trials in 2018
Education
- On-the-job training for new staf
- Support educational programs for clinical trial methodology and GCP (Good Clinical Practice) in the NCC
- Clinical Trial Education programs for other institutions' investigators.
Future prospects
- Increasing clinical trial support capabilities.