Annual Report 2018
Division of Cancer Biology
Hirofumi Arakawa, Yasuyuki Nakamura, Hidefumi Suzuki, Takahiro Shibata, Yoko Sagami, Katsuko Honjo, Makoto Yamamoto, Katsuya Chinen, Naoki Ikari
Research activities
1. Mieap-regulated mitochondrial quality control
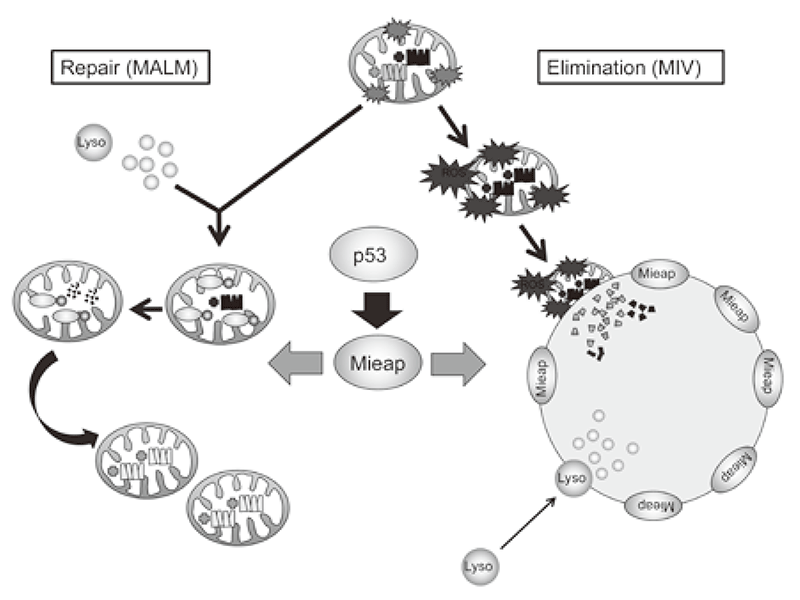
MMieap controls mitochondrial quality via two distinct novel mechanisms. One of the mechanisms has been designated MALM for Mieap-induced accumulation of lysosome-like organelles within mitochondria (PLoS ONE 6: e16054, 2011). In this mechanism, Mieap induces the accumulation of intramitochondrial lysosomal proteins in order to eliminate oxidized mitochondrial proteins in response to mitochondrial damage. This leads to a decrease in reactive oxygen species generation and an increase in mitochondrial ATP synthesis activity, implying MALM plays a role in repairing unhealthy mitochondria.
BNIP3 and NIX, mitochondrial outer membrane proteins, two Mieap-interacting proteins mediate the translocation of lysosomal proteins from cytosol into mitochondria during MALM by forming an unknown pore in the mitochondrial double membrane (PLoS ONE 7: e30767, 2012). 14-3-3γ mediates the degradation of oxidized mitochondrial proteins within mitochondria during MALM (Scientific Reports 2: 379, 2012).
Alternatively, the other mechanism has been designated MIV for Mieap-induced vacuole (PLoS ONE 6: e16060, 2011). When MALM is inhibited, Mieap induces a vacuole-like structure, MIV. The MIV engulfs the damaged mitochondria and accumulates lysosomes, leading to the degradation of unhealthy mitochondria. MIV likely represents a novel mechanism for mitochondrial autophagy, also called "mitophagy". Therefore, Mieap controls mitochondrial quality by repairing or eliminating unhealthy mitochondria via MALM or MIV generation, respectively (Figure 1).
Figure 1. Mieap-regulated mitochondrial quality control

2. Mieap-regulated mitochondrial quality control is frequently inactivated in human cancer
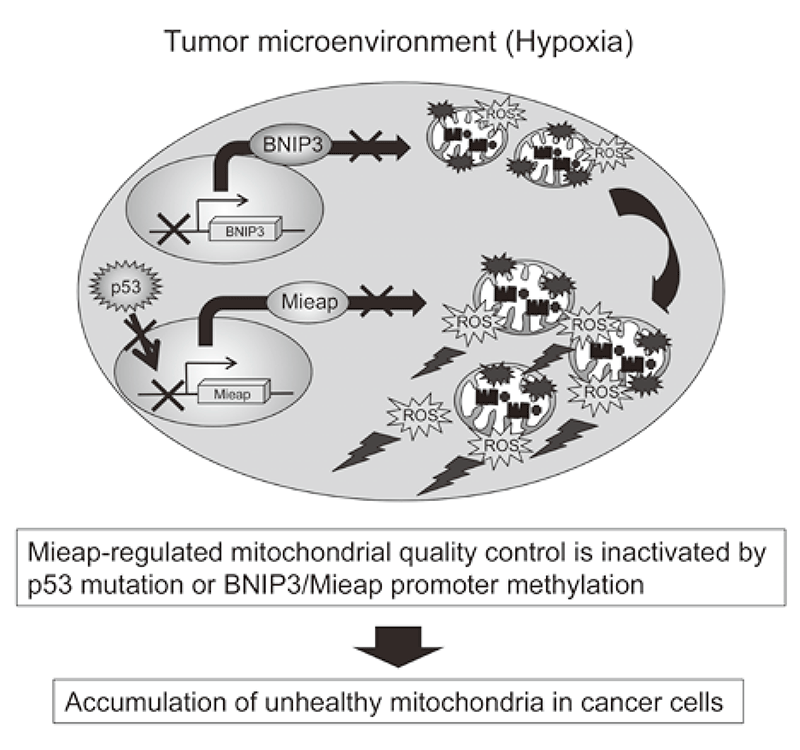
The accumulation of unhealthy mitochondria results in mitochondrial dysfunction, which has been implicated in aging, degenerative diseases and cancer. The Mieap-regulated mitochondrial quality control (MQC) was found to be frequently inactivated by p53 mutations or Mieap/BNIP3 promoter methylation in more than 70% of primary cancer tissues of colorectal cancer patients, leading to accumulation of unhealthy mitochondria and a high level of mitochondria reactive oxygen species generation (ROS) (Oncogenesis 5: e181, 2016). The Mieap-regulated MQC was also inactivated in 26% of primary cancer tissues of breast cancer patients (Cancer Science 109: 3910-3920, 2018).
The elevated mitochondrial ROS causes oxidative damage to DNA, RNA, protein and lipid and so on. This induces genomic instability. The mitochondrial ROS contribute to tumor growth, epithelial-to-mesenchymal transition, cancer invasion, cancer metastasis, tumor angiogenesis through the activation of HIF1, NF-kB, MMPs, AKT, Erk1/2, JNK and so on. Therefore, the Mieap-regulated mitochondrial quality control is a tumor suppressor for colorectal cancer (Figure 2).
Figure 2. Alteration of Mieap-regulated mitochondrial quality control in cancer

3. Mieap-deficient cancer animal model
To clarify the in vivo role of the Mieapregulated MQC in tumorignenesis, the Mieap knockout mice were generated in the Division. Using the Mieap knockout mice, the Mieapdeficient ApcMin/+ mice were also generated and analyzed in order to elucidate the role of Mieap in colorectal cancer tumorigenesis (Scientific Reports 5: 12472, 2015).
Interestingly, the Mieap-deficient ApcMin/+ mice exhibited remarkably reduced lifespans compared with those of ApcMin/+ mice. Furthermore, a substantial increase in the number and size of intestinal polyps was found in the Mieap-deficient ApcMin/+ mice. Histopathologically, intestinal tumors in the Mieap-deficient ApcMin/+ mice clearly exhibited advanced grades of adenoma and adenocarcinomas. Unhealthy mitochondria dramatically accumulated in the tumor cells and generated high level of ROS in the Mieapdeficient ApcMin/+ mice.
We further obtained the same results from the Mieap-deficient gastric cancer model mice. These results suggest that the Mieapregulated MQC pathway has a critical role in the suppression of intestinal tumors in vivo.
4. Non-canonical mitophagy induced by Mieap and its role in tumor suppression via cell death
Parkin/Pink1-mediated mitophagy plays a critical role in mitochondrial quality control, in which the damaged mitochondria are sequestered by autophagosomes, and degraded by fusion between autophagosomes and lysosomes. We found that Mieap-mediated noncanonical mitophagy as a new function of tumor suppressor p53. Mieap induces large vacuoles in cancer cells. Mieap-induced vacuoles are generated from the mitochondria, and directly eat and degrade the cancer mitochondria. Mieapmediated non-canonical mitophagy induces cell death via the iron-dependent production of reactive oxygen species (ROS). Mieap-mediated non-canonical mitophagy plays a critical role in tumor suppression via iron-dependent cell death.
Future prospects
Analyses of cancer cell lines, clinical cancer tissues, and cancer-mouse models enable us to understand the actual role of the Mieap-regulated mitochondrial quality control in human cancer initiation, progression, invasion and metastasis. Finally, we will be able to establish a solid foundation for development of new strategies for cancer prevention, diagnosis, and therapy in the future.
List of papers published in 2018
Journal
1. Gaowa S, Futamura M, Tsuneki M, Kamino H, Tajima JY, Mori R, Arakawa H, Yoshida K. Possible role of p53/Mieap-regulated mitochondrial quality control as a tumor suppressor in human breast cancer. Cancer Sci, 109:3910-3920, 2018
