Annual Report 2018
Department of Omics Network
Masaru Katoh
Introduction
The Department of Omics Network is involved in innovation based on the balance between its main world-class projects and cutting-edge new projects. The FGF (PMID: 23696246), Hedgehog (PMID: 19860666) and WNT (PMID: 17634527) signaling cascades and the Forkhead-box (FOX) family of transcription factors (PMID: 23022474) have been the main (fundamental) projects of the Department. Angiogenesis, cell adhesion, epigenetics and immune evasion in the tumor microenvironment have been new (cutting-edge) projects of the Department. To maintain competitiveness in the global scientific community with continuous and dynamic changes, the Department is currently concentrating its resources on the FGF and WNT signaling cascades.
Masaru Katoh was engaged in clinical medicine from 1986 to 1990, basic medicine from 1990 to 2002 and information science from 2003 to 2011. Since 2012, Katoh has been engaged in the Knowledgebase Project to move toward omicsbased precision medicine.
FGFR-targeted therapy
FGFRs are receptor tyrosine kinases with a role in several biological processes, such as the regulation of development and tissue repair. However, alterations in FGFR1, FGFR2, FGFR3 and FGFR, such as amplifications, fusions and mutations, as well as aberrant epigenetic or transcriptional regulation and changes in tumor-stromal interactions in the tumor microenvironment, can lead to the development and/or progression of cancer. Similar to other kinase alterations, such alterations are targetable using small molecules or antibodies, and the benefits of FGFR inhibitors have been demonstrated in clinical trials involving subsets of patients with solid tumors harboring FGFR alterations. However, the response rates in patients with FGFR alterations were relatively low, and responses in patients without detectable FGFR alterations were also observed. The selection of patients who are most likely to benefit from treatment using RNA in situ hybridization and the use of FGFR inhibitors in combination regimens with other agents might lead to improved response rates and/or the avoidance of acquired resistance.
Immuno-oncology therapy
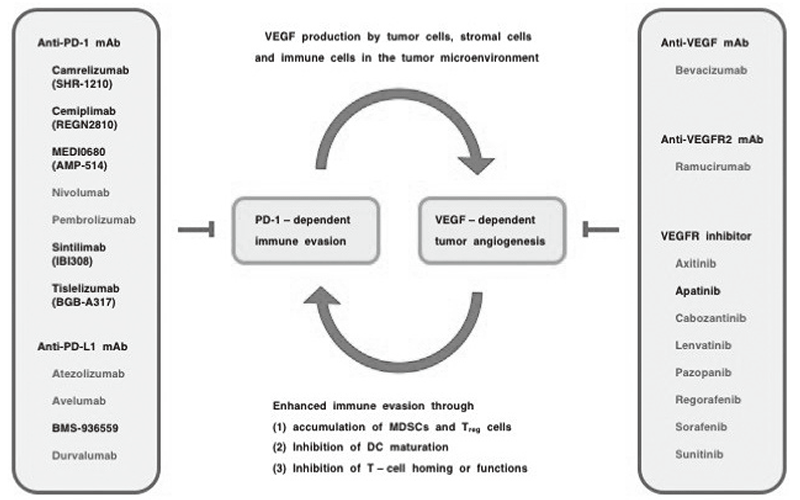
Anti-PD-1 monoclonal antibodies (cemiplimab, nivolumab and pembrolizumab), anti-PD-L1 mAbs (atezolizumab, avelumab, durvalumab) and an anti-CTLA4 mAb (ipilimumab) are immune checkpoint inhibitors that are approved by the US Food and Drug Administration (FDA) for the treatment of cancer patients. However, because the benefits of immune checkpoint inhibitors are limited to a fraction of cancer patients, combination with therapeutics targeting the tumor microenvironment is a rational strategy. Aberrant VEGF signaling in the tumor microenvironment leads to a leaky and hypoxic condition that promotes the survival of cancer stem cells, the epithelial-to-mesenchymal transition of tumor cells, and immune evasion through the recruitment of myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells and the functional suppression of CD8+ T cells and natural killer (NK) cells. AntiVEGF mAb (bevacizumab), anti-VEGFR2 mAb (ramucirumab) and VEGFR inhibitors (axitinib, cabozantinib, lenvatinib, pazopanib, regorafenib, sorafenib and sunitinib) are VEGF signaling targeted therapeutics. The combinatorial optimization of immune checkpoint inhibitors and VEGF signaling blockers (Figure 1) as well as the development of biomarkers for the positive and negative selection of patients are necessary for the beneficial maximization of immunooncology drugs for the treatment of cancer patients.
Figure 1. Combination immuno-oncology therapy with VEGF signaling blockers.

Contribution to the global scientific community
Masaru Katoh has been contributing to the global scientific community through manuscript publication, reviewer activity and editor activity. Katoh carried out peer reviews of grant proposals or journal manuscripts written in English 63 times in 2018. Katoh is a member of the International Expert Panel of the National Medical Research Council (NMRC) of Singapore. Katoh is an Academic Editor of a USA-based journal, PLoS ONE, and carried out editorial decisions 86 times in 2018. Katoh is the Chief Editor of a Switzerland-based journal, Frontiers in Molecular Medicine, that aims to address the gap between cell and developmental biology and clinical medicine, together with 220 editorial board members.
The manuscript citation count in the Web of Science Database is a surrogate marker of the contribution to the global scientific community. Katoh's manuscripts were cited approximately 600 times by others in 2018.
List of papers published in 2018
Journal
1. Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol, 16:105-122, 2019
2. Katoh M. Multilayered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/betacatenin signaling activation (Review). Int J Mol Med, 42:713-725, 2018
3. Katoh M. Combination immuno-oncology therapy with pembrolizumab, an anti-PD-1 monoclonal antibody targeting immune evasion, and standard chemotherapy for patients with the squamous and non-squamous subtypes of non-small cell lung cancer. J Thorac Dis, 10:5178-5183, 2018
