Annual Report 2019
Department of Hepatobiliary and Pancreatic Oncology
Masafumi Ikeda, Shuichi Mitsunaga, Hiroshi Imaoka, Yusuke Hashimoto, Mitsuhito Sasaki, Kazuo Watanabe, Shoichi Miyazawa, Toru Otsuru, Taro Shibuki, Yasutaka Shimotsuura, Kohei Hayashi
Introduction
The Department of Hepatobiliary and Pancreatic Oncology is responsible for the diagnosis and treatment of patients with hepatic, biliary, and pancreatic cancers as well as interventional management by endoscopic or percutaneous procedures (Table 1). Our goal is to provide high-quality cancer treatment with adequate palliative care, and to develop novel and effective treatments and procedures through well-designed clinical trials and research.
Table 1. Number of cancer patients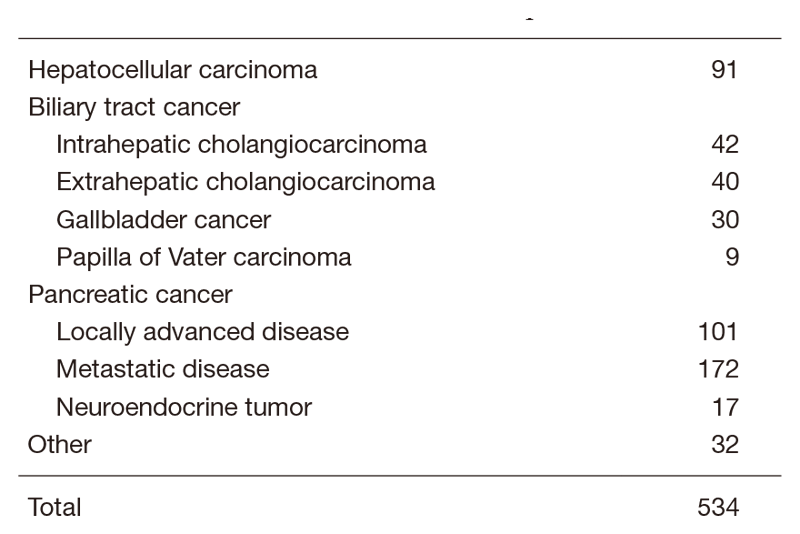

The Team and What We Do
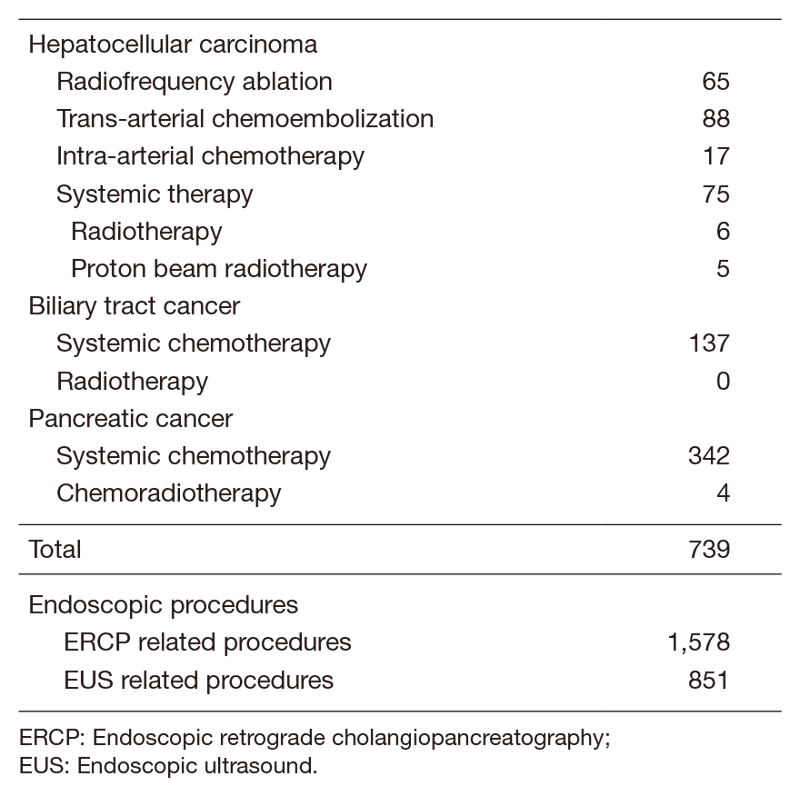
Our department is composed of seven staff oncologists and four residents, with an average of 44 beds in the hospital. These doctors are divided into two teams and each team conducts clinical rounds for each admitted patient every morning and evening. The treatment strategies for individual patients are discussed in weekly tumor board conferences attended by medical oncologists, surgeons, radiologists, radiation oncologists, and pharmacists. Furthermore, we are also responsible for endoscopic abdominal ultrasonographic examinations, endoscopic or percutaneous ultrasound-guided biopsies of abdominal masses, local ablative therapy for liver tumors, endoscopic or percutaneous biliary drainage, abscess drainage and stenting for obstructive jaundice, etc. (Table 2).
Research activities
Hepatocellular carcinoma (HCC)
Trans-arterial chemoembolization (TACE) is the only guideline-recommended global standard of care for intermediate-stage HCC. However, not all patients benefit from TACE because intermediate-stage HCC is a heterogeneous disease in terms of tumor burden and liver function. In intermediate-stage HCC, we proposed the concept of “TACE unsuitable” with Asian HCC experts and announced it at the Asia-Pacific Primary Liver Cancer Expert (APPLE) Consensus Statement. Following that, some clinical trials being planned for “TACE unsuitable” patients.
The combination therapy of immune-checkpoint inhibitors plus VEGF inhibitors has some promising efficacy for unresectable HCC. Atezolizumab plus bevacizumab demonstrated statistically significant and clinically meaningful improvement of overall survival, progression free survival, objective response rate and patient-reported quality of life over sorafenib. Therefore, it should be considered a practice-changing treatment for patients with HCC who have not received prior systemic therapy, and is expected to be reimbursed in Japan in 2020. Pembrolizumab plus lenvatinib also demonstrated favorable tumor shrinkage effects in advanced HCC in an international phase Ib trial. A global phase III trial of pembrolizumab plus lenvatinib vs. placebo plus lenvatinib (LEAP-002) is ongoing. We are deeply involved in these trials.
Biliary tract cancer (BTC)
Monotherapy of immune-checkpoint inhibitors have a limited efficacy for advanced BTC, but bintrafusp alfa, which was a bifunctional fusion protein targeting TGF-β and PD-L1, has been shown to have a higher response rate. At present, a phase II trial of monotherapy with bintrafusp alfa is underway as a second-line treatment for BTC patients. The other immune-checkpoint inhibitors are under development as combination therapies with gemcitabine (Gem) plus cisplatin therapy or in combination with other agents.
Pancreatic cancer (PC)
Gem plus nab-paclitaxel and modified FOLFIRINOX has been often administered as a first-line treatment for advanced PC. Also, S-IROX, which is a regimen that replaces 5-fluorouracil (5-FU) of mFOLFIRNOX with S-1, has a favorable tumor response rate for advanced PC. Therefore, a phase III trial of Gem plus nab-paclitaxel vs. modified FOLFIRINOX vs. S-IROX (JCOG1611) is ongoing as a first-line setting.
Nano-liposomal irinotecan (nalIRI) plus 5-FU/leucovorin (LV) has significantly increased overall survival vs. 5 FU/LV in patients with metastatic PC that progressed on prior Gem-based therapy. The results of a Japanese phase II trial of nalIRI+5-FU/LV have been also published, and it is expected to be reimbursed in Japan in 2020. We are planning to develop a regimen that replaces 5-FU of NalIRI + 5-FU / LV with S-1.
Other research
In 107 patients with liver metastasis of PC, tumors showing the replacement growth pattern, in which metastatic cells infiltrate and replace hepatocytes with minimal desmoplastic reaction and inflammatory cell infiltration, were identified as being associated with a poor prognosis.
Clinical trials
Seventy-two clinical trials (sponsored: 42 trials, investigator-initiated: 30 trials) are ongoing, and 12 clinical trials (sponsored: eight trials, investigator-initiated: five trials) are being planned for the upcoming year.
HCC
The enrollment of a multicenter phase II trial of lenvatinib plus intra-arterial cisplatin and a randomized phase II trial comparing trans-arterial chemoembolization (TACE) using drug eluting beads vs. conventional TACE (PRESIDENT) were terminated, and the final analysis will be opened in the near future. Some sponsored trials of pembrolizumab plus lenvatinib and atezolizumab plus bevacizumab, as the first-line chemotherapy, were closed. A global phase III trial of ipilimumab plus nivolumab vs. sorafenib, as the first line therapy, and a phase I trial of lenvatinib plus beta-catenin modulator, as the second-line therapy, are ongoing.
BTC
An investigator-initiated trial of FOLFIRINOX and some sponsored trials of GC plus immune-checkpoint inhibitors, including durvalumab, bintrafusp alfa and pembrolizumab, as first-line chemotherapy were ongoing. Some sponsored trials of resminostat plus S-1, capecitabine plus varlitinib, BAY1436032, and bintrafusp alfa, for advanced BTCs refractory to Gem, closed recruitment. Some sponsored trials of JPH203, nivolumab plus lenvatinib, E7090 for FGFR2 fusion, DS8201a for HER2 positive, etc., for advanced BTCs refractory to Gem, were conducted. For patients with occupational cholangiocarcinoma, a phase II trial of nivolumab was ongoing.
PC
Some clinical trials of a phase II/III trial of neoadjuvant S-1 and concurrent radiotherapy vs. Gem plus nab-paclitaxel for borderline resectable PC (GABARNANCE), Gem plus nab-paclitaxel vs. modified FOLFIRINOX vs. S-IROX for metastatic PC (JCOG1611), as the first-line setting, were ongoing. The enrollment of some sponsored trials of HF10 plus Gem plus nab-paclitaxel, modified FOLFIRINOX plus nivolumab as the first-line setting, and cabiralizumab plus nivolumab as the second-line setting, were terminated. An investigator-initiated trial of phase I trial of tocilizumab plus Gem plus nab-paclitaxel was initiated and the enrollment has already finished.
Education
For our residents, daily training is provided through group discussions on the daily practice of management of inpatients and outpatients. Also, they can learn about the indications, administration and management of the adverse events from loco-regional treatments to systemic chemotherapy for hepatic, biliary, and pancreatic cancer patients and the accompanying procedures to undertake diagnosis and interventional management, and so on. In addition, they can give presentations on their research in domestic and overseas meetings and write papers in English under the instruction of staff physicians.
Future prospects
The prognosis of patients with hepatic, biliary, and pancreatic cancers remains dismal, and the efficacy of standard treatments for these cancers is limited. In Japan, the incidences of these cancers, especially HCC and BTC, are higher than in Western countries. Therefore, we must conduct a lot of top-level novel and promising clinical trials and research. It is also necessary to develop biomarker research and endoscopic management alongside cancer treatment.
