Annual Report 2019
Department of General Internal Medicine
Toshihiko Doi, Keiji Okinaka, Yoichi Naito, Yasutoshi Kuboki, Yusuke Hashimoto, Tomofumi Miura, Takaya Ikeda, Kensuke Shinmura, Nobuhiko Yamauchi
Introduction
1. General internal medicine
We provide general management across cancer types, support for medical treatment in each department (management for complications, adverse events, etc.), and management aimed at training oncologic specialists. As an educational hospital of the Japanese Society of Internal Medicine, we contribute to training for internal medicine with the aim of becoming a general medical specialist. Based on the experience of immune-related adverse events (irAEs) associated with increasing indications and use of immune checkpoint inhibitors in a wide range of diseases, we work with all the departments, including the Department of Pharmacy and Department of Nursing to recognize the occurrence of irAEs and take countermeasures. We also assist research related to irAEs.
2. Infectious diseases
The mission of the infectious diseases section is to provide consultation on clinical infectious diseases. We also work with the Office of Infection Control and Prevention to prevent healthcare-associated infections during cancer care.
The Team and What We Do
1. General internal medicine
- Increased number of reported irAEs (Figure 1)
- Implementation of irAE collaboration seminar (twice: fiscal year 2019)
- Development of a manual for immune checkpoint inhibitors
Figure 1. Reported Number of irAE Cases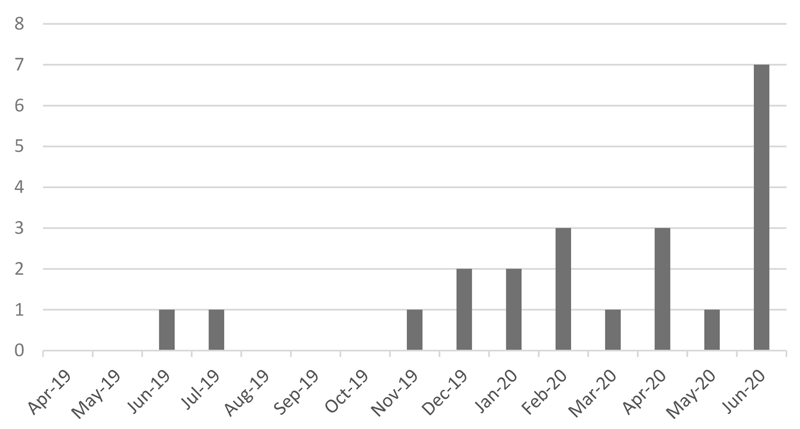

2. Infectious diseases
We provided 390 infectious disease consultations in this period (Table 1) and also promote hospital infection control. (See the “Office of Infection Control and Prevention” section.)
The number of cases managed during this period:
- Positive blood culture cases: 330
- Cases using broad-spectrum antibiotics: 1,755
Table 1. Number of infectious disease consultations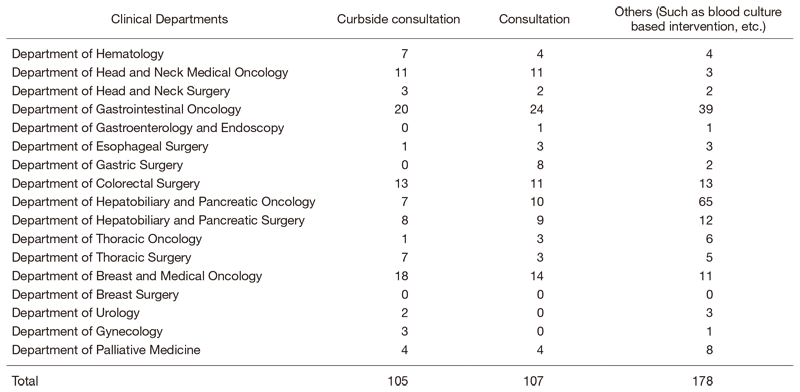

Research activities
Case presentation support for trainees at the congress of the Japanese Society of Internal Medicine
- Paper writing support (Example: Inoue M, Naito Y, et al.; Ann Case Report 2020; 14: 416.)
- Intensive lectures on medical oncology education for new residents (recorded, updated on the web)
- Support/guidance for new research (Example: Observational study to confirm pharmacokinetics of mycophenolate mofetil for liver damage caused by immune checkpoint inhibitors)
- Contributing to training for internal medicine trainees
- irAE cooperation seminar with regional institutions
Clinical trials
One observational study is currently planned by the Department of Pharmacy, and is a study that we back up entirely
Education
We will further collaborate with regional institutions to assemble outstanding trainees, and develop tight connections with several academic institutions. We will prepare a manual for immune checkpoint inhibitors, and develop safe and cost-effective practice. We plan to have approximately 100 case consultations on immune related AEs.
Future prospects
1. General internal medicine
We will continue high-quality training. The medical treatment for cancer at our hospital is at the top level in Japan, and we will continue to improve the medical treatment system so that the valuable experience gained at this hospital can be returned to general medical treatment, and to establish a consulting system. We will continue the irAE cooperation program and promote research planning for issue identification and improvement. We will deepen cooperation with the Department of Pharmacy and Department of Nursing, and continue efforts to share and improve issues within the hospital. Regarding education, we will also cooperate with the Human Resource Development Center to enhance internal education and will continuously cooperate with external parties. We will continuously confirm that medical issues can be shared with the risk management department.
2. Infectious Diseases
Consultation services for infectious diseases are now increasingly recognized as key components of cancer centers, some of which have begun to establish a department for infectious diseases. The future goal is to launch fellowship programs for fellows to develop high-level expertise and assume a key role in this field.
List of papers published in 2019
Journal
1. Hatogai K, Fujii S, Kitano S, Kojima T, Daiko H, Yoshino T, Ohtsu A, Takiguchi Y, Doi T, Ochiai A. Relationship between the immune microenvironment of different locations in a primary tumour and clinical outcomes of oesophageal squamous cell carcinoma. Br J Cancer, 122:413-420, 2020
2. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol, 38:1-10, 2020
3. Shinmura K, Ikematsu H, Kojima M, Nakamura H, Osera S, Yoda Y, Hori K, Oono Y, Ochiai A, Yano T. Safety of endoscopic procedures with monopolar versus bipolar instruments in an ex vivo porcine model. BMC Gastroenterol, 20:27, 2020
4. Kogawa T, Fujii T, Wu J, Harano K, Fouad TM, Liu DD, Shen Y, Masuda H, Krishnamurthy S, Chavez-MacGregor M, Lim B, Murthy RK, Valero V, Tripathy D, Ueno NT. Prognostic Value of HER2 to CEP17 Ratio on Fluorescence In Situ Hybridization Ratio in Patients with Nonmetastatic HER2-Positive Inflammatory and Noninflammatory Breast Cancer Treated with Neoadjuvant Chemotherapy with or without Trastuzumab. Oncologist, 25:e909-e919, 2020
5. Minamide T, Yoda Y, Hori K, Shinmura K, Oono Y, Ikematsu H, Yano T. Advantages of salvage photodynamic therapy using talaporfin sodium for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Surg Endosc, 34:899-906, 2020
6. Sato D, Motegi A, Kadota T, Kojima T, Bando H, Shinmura K, Hori K, Yoda Y, Oono Y, Zenda S, Ikematsu H, Akimoto T, Yano T. Therapeutic results of proton beam therapy with concurrent chemotherapy for cT1 esophageal cancer and salvage endoscopic therapy for local recurrence. Esophagus, 17:305-311, 2020
7. Kadota T, Yoda Y, Hori K, Shinmura K, Oono Y, Ikematsu H, Yano T. Prophylactic steroid administration against strictures is not enough for mucosal defects involving the entire circumference of the esophageal lumen after esophageal endoscopic submucosal dissection (ESD). Esophagus, 2020
8. Higashibata T, Tagami K, Miura T, Okizaki A, Watanabe YS, Matsumoto Y, Morita T, Kinoshita H. Usefulness of painDETECT and S-LANSS in identifying the neuropathic component of mixed pain among patients with tumor-related cancer pain. Support Care Cancer, 28:279-285, 2020
9. Kato H, de Souza P, Kim SW, Lickliter JD, Naito Y, Park K, Kumar S, Mugundu GM, Bang YJ. Safety, Pharmacokinetics, and Clinical Activity of Adavosertib in Combination with Chemotherapy in Asian Patients with Advanced Solid Tumors: Phase Ib Study . Target Oncol, 15:75-84, 2020
10. Naito Y, Mishima S, Akagi K, Igarashi A, Ikeda M, Okano S, Kato S, Takano T, Tsuchihara K, Terashima K, Nishihara H, Nishiyama H, Hiyama E, Hirasawa A, Hosoi H, Maeda O, Yatabe Y, Okamoto W, Ono S, Kajiyama H, Nagashima F, Hatanaka Y, Miyachi M, Kodera Y, Yoshino T, Taniguchi H. Japan society of clinical oncology/Japanese society of medical oncology-led clinical recommendations on the diagnosis and use of tropomyosin receptor kinase inhibitors in adult and pediatric patients with neurotrophic receptor tyrosine kinase fusion-positive advanced solid tumors, cooperated by the Japanese society of pediatric hematology/oncology . Int J Clin Oncol, 25:403-417, 2020
11. Naito Y, Kai Y, Ishikawa T, Fujita T, Uehara K, Doihara H, Tokunaga S, Shimokawa M, Ito Y, Saeki T. Chemotherapy-induced nausea and vomiting in patients with breast cancer: a prospective cohort study. Breast Cancer, 27:122-128, 2020
12. Nishida T, Sakai Y, Takagi M, Ozaka M, Kitagawa Y, Kurokawa Y, Masuzawa T, Naito Y, Kagimura T, Hirota S. Adherence to the guidelines and the pathological diagnosis of high-risk gastrointestinal stromal tumors in the real world . Gastric Cancer, 23:118-125, 2020
13. Doi T, Aramaki T, Yasui H, Muro K, Ikeda M, Okusaka T, Inaba Y, Nakai K, Ikezawa H, Nakajima R. A phase I study of ontuxizumab, a humanized monoclonal antibody targeting endosialin, in Japanese patients with solid tumors. Invest New Drugs, 37:1061-1074, 2019
14. Doi T, Iwasa S, Muro K, Satoh T, Hironaka S, Esaki T, Nishina T, Hara H, Machida N, Komatsu Y, Shimada Y, Otsu S, Shimizu S, Watanabe M. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial. Gastric Cancer, 22:817-827, 2019
15. Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, Oizumi S, Gemmoto K, Suna H, Enokitani K, Kawakami T, Nishikawa H, Yamamoto N. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin Cancer Res, 25:6614-6622, 2019
16. Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, Tsurutani J, Kadowaki S, Yamaguchi K, Iwasa S, Saito K, Fujisaki Y, Sugihara M, Shahidi J, Doi T. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol, 20:827-836, 2019
17. Doi T, Yoh K, Shitara K, Takahashi H, Ueno M, Kobayashi S, Morimoto M, Okusaka T, Ueno H, Morizane C, Okano N, Nagashima F, Furuse J. First-in-human phase 1 study of novel dUTPase inhibitor TAS-114 in combination with S-1 in Japanese patients with advanced solid tumors. Invest New Drugs, 37:507-518, 2019
18. Esaki T, Hirai F, Makiyama A, Seto T, Bando H, Naito Y, Yoh K, Ishihara K, Kakizume T, Natsume K, Myers A, Doi T. Phase I dose-escalation study of capmatinib (INC280) in Japanese patients with advanced solid tumors. Cancer Sci, 110:1340-1351, 2019
19. Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Kuwata T, Akimoto T, Shitara K. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer, 22:793-802, 2019
20. Koganemaru S, Kuboki Y, Koga Y, Kojima T, Yamauchi M, Maeda N, Kagari T, Hirotani K, Yasunaga M, Matsumura Y, Doi T. U3-1402, a Novel HER3-Targeting Antibody-Drug Conjugate, for the Treatment of Colorectal Cancer. Mol Cancer Ther, 18:2043-2050, 2019
21. Doi T, Kurokawa Y, Sawaki A, Komatsu Y, Ozaka M, Takahashi T, Naito Y, Ohkubo S, Nishida T. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: a phase II, single-arm trial. Eur J Cancer, 121:29-39, 2019
22. Kotani D, Kuboki Y, Horasawa S, Kaneko A, Nakamura Y, Kawazoe A, Bando H, Taniguchi H, Shitara K, Kojima T, Tsuji A, Yoshino T. Retrospective cohort study of trifluridine/tipiracil (TAS-102) plus bevacizumab versus trifluridine/tipiracil monotherapy for metastatic colorectal cancer. BMC Cancer, 19:1253, 2019
23. Kato K, Shah MA, Enzinger P, Bennouna J, Shen L, Adenis A, Sun JM, Cho BC, Özgüroğlu M, Kojima T, Kostorov V, Hierro C, Zhu Y, McLean LA, Shah S, Doi T. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol, 15:1057-1066, 2019
24. Harding JJ, Bauer TM, Tan DSW, Bedard PL, Rodon J, Doi T, Schnell C, Iyer V, Baffert F, Radhakrishnan R, Fabre C, Juric D. Characterization and phase I study of CLR457, an orally bioavailable pan-class I PI3-kinase inhibitor. Invest New Drugs, 37:271-281, 2019
25. Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, Sagara Y, Doi T, Park H, Murthy RK, Redman RA, Jikoh T, Lee C, Sugihara M, Shahidi J, Yver A, Modi S. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol, 20:816-826, 2019
26. Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, Borg C, Doi T, Yoon HH, Savage MJ, Wang J, Dalal RP, Shah S, Wainberg ZA, Chung HC. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer, 22:828-837, 2019
27. Oono Y, Shinmura K, Hori K, Yoda Y, Ishii G, Ikematsu H, Yano T. Endoscopic submucosal resection using a ligation device without injection for duodenal neuroendocrine tumors. Surg Endosc, 33:2008-2014, 2019
28. Minamide T, Shinmura K, Ikematsu H, Yano T. Early-stage primary signet ring cell carcinoma of the colon with magnifying endoscopic findings. Gastrointest Endosc, 90:529-531, 2019
29. Kumahara K, Ikematsu H, Shinmura K, Murano T, Inaba A, Okumura K, Nishihara K, Sunakawa H, Furue Y, Ito R, Sato D, Minamide T, Okamoto N, Yamamoto Y, Suyama M, Takashima K, Nakajo K, Yoda Y, Hori K, Oono Y, Yano T. Objective evaluation of the visibility of colorectal lesions using eye tracking. Dig Endosc, 31:552-557, 2019
30. Yamamoto Y, Shinmura K, Yano T. Two cases of early gastric and esophageal cancers treated by endoscopic submucosal dissection in three-dimensional endoscopy. Dig Endosc, 31:e120-e121, 2019
31. Murano T, Ikematsu H, Shinmura K, Ito R, Minamide T, Sato D, Yamamoto Y, Takashima K, Kadota T, Yoda Y, Hori K, Oono Y, Yano T. Endoscopic prediction of advanced histology in colorectal lesions sized <10 mm using the Japan Narrow-band imaging Expert Team classification. Dig Endosc, 2019
32. Shitara K, Ueha S, Shichino S, Aoki H, Ogiwara H, Nakatsura T, Suzuki T, Shimomura M, Yoshikawa T, Shoda K, Kitano S, Yamashita M, Nakayama T, Sato A, Kuroda S, Wakabayashi M, Nomura S, Yokochi S, Ito S, Matsushima K, Doi T. First-in-human phase 1 study of IT1208, a defucosylated humanized anti-CD4 depleting antibody, in patients with advanced solid tumors. J Immunother Cancer., 7:195, 2019
33. Doi T, Fujiwara Y, Matsubara N, Tomomatsu J, Iwasa S, Tanaka A, Endo-Tsukude C, Nakagawa S, Takahashi S. Phase I study of ipatasertib as a single agent and in combination with abiraterone plus prednisolone in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 84:393-404, 2019
34. Kimura G, Hashimoto Y, Ikeda M. Endoscopic unroofing drainage with a needle-knife for gastric wall abscess: a rare adverse event that developed after EUS-FNA. VideoGIE, 4:512-513, 2019
35. Imaoka H, Sasaki M, Hashimoto Y, Watanabe K, Ikeda M. New Era of Endoscopic Ultrasound-Guided Tissue Acquisition: Next-Generation Sequencing by Endoscopic Ultrasound-Guided Sampling for Pancreatic Cancer. J Clin Med, 8:1173, 2019
36. Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, Ueno T, Kwong A, Li H, Huang SM, Leung R, Han W, Tan B, Hu FC, Huang CS, Cheng AL, Lu YS. Contrasting Epidemiology and Clinicopathology of Female Breast Cancer in Asians vs the US Population . J Natl Cancer Inst, 111:1298-1306, 2019
37. Shimomura A, Yonemori K, Yoshida M, Yoshida T, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hamada A, Michimae H, Hashimoto J, Yamamoto H, Kawachi A, Shimizu C, Fujiwara Y, Tamura K. Gene Alterations in Triple-Negative Breast Cancer Patients in a Phase I/II Study of Eribulin and Olaparib Combination Therapy . Transl Oncol, 12:1386-1394, 2019
38. Kobayashi E, Naito Y, Asano N, Maejima A, Endo M, Takahashi S, Megumi Y, Kawai A. Interim results of a real-world observational study of eribulin in soft tissue sarcoma including rare subtypes . Jpn J Clin Oncol, 49:938-946, 2019
39. Yeo W, Ueno T, Lin CH, Liu Q, Lee KH, Leung R, Naito Y, Park YH, Im SA, Li H, Yap YS, Lu YS. Treating HR+/HER2- breast cancer in premenopausal Asian women: Asian Breast Cancer Cooperative Group 2019 Consensus and position on ovarian suppression . Breast Cancer Res Treat, 177:549-559, 2019
