Annual Report 2019
Department of Experimental Therapeutics
Noboru Yamamoto, Toshio Shimizu, Kan Yonemori, Shigehisa Kitano, Shunsuke Kondo, Satoru Iwasa, Tatsuya Yoshida, Kazuki Sudo, Takafumi Koyama, Jun Sato
Introduction
In April 2015, the affiliation of the Department of Experimental Therapeutics was updated from the NCC-EPOC (cf. the Exploratory Oncology Research & Clinical Trial Center) to the NCC-Hospital. The goal of this department is to perform initial clinical evaluation of promising new anti-cancer compounds emerging from the laboratory in phase I trials. The staff consists of specialists from various oncology fields (i.e., thoracic oncology, breast & medical oncology, gastro-intestinal oncology, hepato-biliary & pancreatic oncology, and immuno-oncology).
The Team and What We Do
This department plays a key role in new anti-cancer drug development in Japan as well as in Asia. The top priority is to conduct FIH trials, and we also perform phase I trials for solid tumors (i.e., all comers). Recently, we have joined the global phase I trials to accelerate the new drug development in Japan. Web- or tele-conferences are held with the EU and US sites, and we have been discussing patient enrollment as well as further developmental strategies. Routine web-conferences are also held between the NCC-Hospital (Tokyo) and NCC-East hospital (Chiba) every Friday morning, and we share information about adverse events and patient enrollment and refer the candidates to each other to accelerate enrollment. Nowadays, most of the phase I trials in Japan (i.e., first in Japan phase I trials, first in human trials) are conducted at the NCC-Hospital and NCC-East hospital with firm collaboration.
Research activities
The elucidation of the proof of concept (POC) is essential in new anti-cancer drug development especially in early phases, so we conduct several translational research (TR) projects in collaboration with research institutes. Also, we are conducting TR with the pharmaceutical industry to discover new targets for anti-immune therapy using human tissue (tumor and normal tissue) samples. To facilitate the POC analysis in the phase I trials, a phase I biobank study was launched in 2018.
Clinical trials
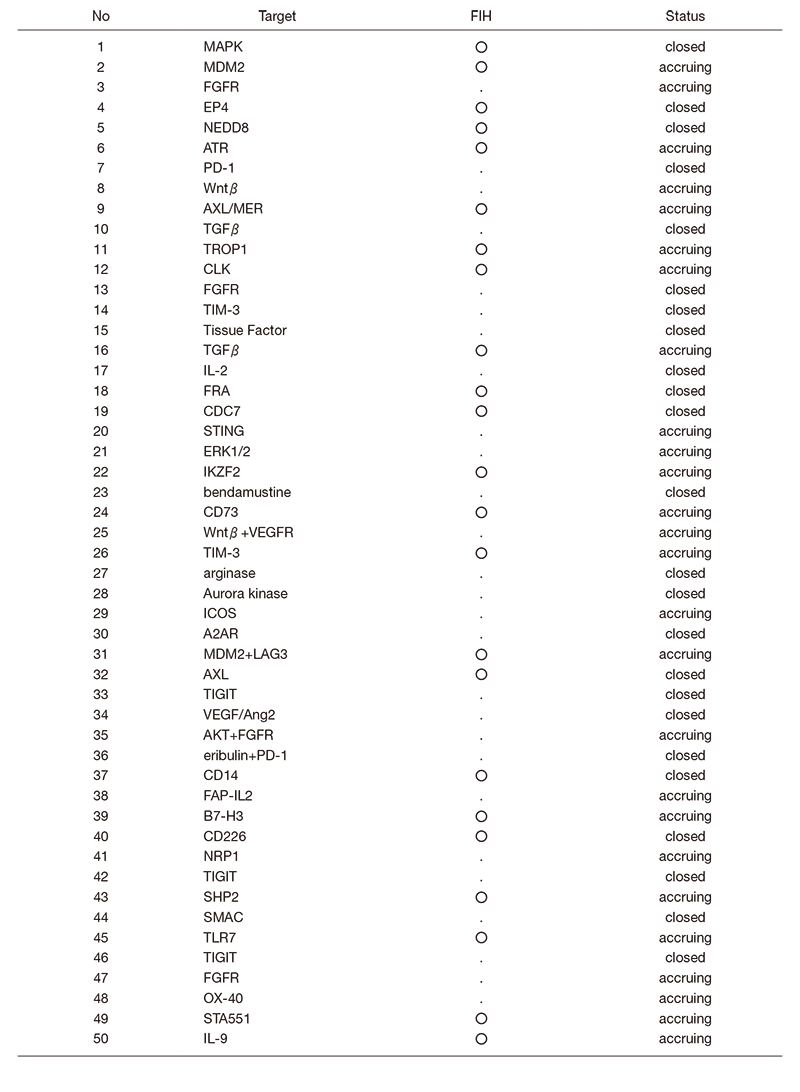
In fiscal 2019, 50 phase I trials including 23 FIH trials were conducted (Table 1).
Education
In 2019, the 4th NCCH Workshop on Methods in Oncology Phase I trials and Translational Research was held on 28 July at the NCC-Tsukiji campus.
List of papers published in 2019
Journal
1. Yamamoto Y, Kondo S, Matsuzaki J, Esaki M, Okusaka T, Shimada K, Murakami Y, Enomoto M, Tamori A, Kato K, Aoki Y, Takizawa S, Sakamoto H, Niida S, Takeshita F, Ochiya T. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients With Liver Disease. Hepatol Commun, 4:284-297, 2020
2. Honma Y, Nagashima K, Hirano H, Shoji H, Iwasa S, Takashima A, Okita N, Kato K, Boku N, Murakami N, Inaba K, Ito Y, Itami J, Kanamori J, Oguma J, Daiko H. Clinical outcomes of locally advanced esophageal neuroendocrine carcinoma treated with chemoradiotherapy. Cancer Med, 9:595-604, 2020
3. Doi T, Matsubara N, Kawai A, Naka N, Takahashi S, Uemura H, Yamamoto N. Phase I study of TAS-115, a novel oral multi-kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs, 38:1175-1185, 2020
4. Ebata T, Shimizu T, Fujiwara Y, Tamura K, Kondo S, Iwasa S, Yonemori K, Shimomura A, Kitano S, Koyama T, Sato N, Nakai K, Inatani M, Yamamoto N. Phase I study of the indoleamine 2,3-dioxygenase 1 inhibitor navoximod (GDC-0919) as monotherapy and in combination with the PD-L1 inhibitor atezolizumab in Japanese patients with advanced solid tumours. Invest New Drugs, 38:468-477, 2020
5. Yamamoto N, Ryoo BY, Keam B, Kudo M, Lin CC, Kunieda F, Ball HA, Moran D, Komatsu K, Takeda K, Fukuda M, Furuse J, Morita S, Doi T. A phase 1 study of oral ASP5878, a selective small-molecule inhibitor of fibroblast growth factor receptors 1-4, as a single dose and multiple doses in patients with solid malignancies. Invest New Drugs, 38:445-456, 2020
6. Ebata T, Shimizu T, Koyama T, Shimomura A, Iwasa S, Kondo S, Kitano S, Yonemori K, Fujiwara Y, Yamamoto N. Improved survival among patients enrolled in oncology phase 1 trials in recent decades. Cancer Chemother Pharmacol, 85:449-459, 2020
7. Frezza AM, Assi T, Lo Vullo S, Ben-Ami E, Dufresne A, Yonemori K, Noguchi E, Siontis B, Ferraro R, Teterycz P, Duffaud F, Ravi V, Vincenzi B, Gelderblom H, Pantaleo MA, Baldi GG, Desar I, Fedenko A, Maki RG, Jones RL, Benjamin RS, Blay JY, Kawai A, Gounder M, Gronchi A, Le Cesne A, Mir O, Czarnecka AM, Schuetze S, Wagner AJ, Adam J, Barisella M, Sbaraglia M, Hornick JL, Meurgey A, Mariani L, Casali PG, Thornton K, Stacchiotti S. Systemic treatments in MDM2 positive intimal sarcoma: A multicentre experience with anthracycline, gemcitabine, and pazopanib within the World Sarcoma Network. Cancer, 126:98-104, 2020
8. Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, Hoshina Y, Sakata Y, Takahashi TY, Nakashima K, Nakao M, Takei D, Zenda S, Mizukami K, Iwasa S, Sakurai M, Yamamoto N, Ohe Y. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 21:242-249, 2020
9. Ida H, Goto Y, Sato J, Kanda S, Shinno Y, Morita R, Murakami S, Matsumoto Y, Yoshida T, Horinouchi H, Fujiwara Y, Yamamoto N, Fukuda T, Ohashi K, Ohe Y. Clinical characteristics of adrenal insufficiency as an immune-related adverse event in non-small-cell lung cancer. Med Oncol, 37:30, 2020
10. Ito A, Kitano S, Tajima K, Kim Y, Tanaka T, Inamoto Y, Kim SW, Yamamoto N, Fukuda T, Okamoto S. Impact of low-dose anti-thymocyte globulin on immune reconstitution after allogeneic hematopoietic cell transplantation. Int J Hematol, 111:120-130, 2020
11. Ito M, Fujiwara Y, Kubo T, Matsushita H, Kumamoto T, Suzuki T, Sunami K, Yamamoto N, Kohno T. Clonal Hematopoiesis From Next Generation Sequencing of Plasma From a Patient With Lung Adenocarcinoma: A Case Report. Front Oncol, 10:113, 2020
12. Kang YK, Bang YJ, Kondo S, Chung HC, Muro K, Dussault I, Helwig C, Osada M, Doi T. Safety and Tolerability of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Asian Patients with Pretreated Recurrent or Refractory Gastric Cancer. Clin Cancer Res, 26:3202-3210, 2020
13. Kato MK, Yunokawa M, Bun S, Shimoi T, Yonemori K, Miyasaka N, Kato T, Tamura K. Treatment strategies for recurrent ovarian cancer in older adult patients in Japan: a study based on real-world data. J Cancer Res Clin Oncol, 146:1335-1341, 2020
14. Kawachi A, Yamashita S, Okochi-Takada E, Hirakawa A, Tsuda H, Shimomura A, Kojima Y, Yonemori K, Fujiwara Y, Kinoshita T, Ushijima T, Tamura K. BRCA1 promoter methylation in breast cancer patients is associated with response to olaparib/eribulin combination therapy. Breast Cancer Res Treat, 181:323-329, 2020
15. Koyama T, Shimizu T, Iwasa S, Fujiwara Y, Kondo S, Kitano S, Yonemori K, Shimomura A, Iizumi S, Sasaki T, Furuse J, Yamamoto N. First-in-human phase I study of E7090, a novel selective fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. Cancer Sci, 111:571-579, 2020
16. Mizuta H, Nakano E, Takahashi A, Koyama T, Namikawa K, Yamazaki N. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: A case report and literature review. Dermatol Ther, 33:e13321, 2020
17. Nakatani Y, Kato K, Shoji H, Iwasa S, Honma Y, Takashima A, Ushijima T, Ito Y, Itami J, Boku N. Comparison of involved field radiotherapy and elective nodal irradiation in combination with concurrent chemotherapy for T1bN0M0 esophageal cancer. Int J Clin Oncol, 25:1098-1104, 2020
18. Niho S, Yoshida T, Akimoto T, Sakamaki K, Ono A, Seto T, Nishio M, Yamamoto N, Hida T, Okamoto H, Kurata T, Satouchi M, Goto K, Yamanaka T, Ohe Y. Randomized phase II study of chemoradiotherapy with cisplatin + S-1 versus cisplatin + pemetrexed for locally advanced non-squamous non-small cell lung cancer: SPECTRA study. Lung Cancer, 141:64-71, 2020
19. Okuma HS, Yonemori K, Narita SN, Sukigara T, Hirakawa A, Shimizu T, Shibata T, Kawai A, Yamamoto N, Nakamura K, Nishida T, Fujiwara Y. MASTER KEY Project: Powering Clinical Development for Rare Cancers Through a Platform Trial. Clin Pharmacol Ther, 2020
20. Otsuka R, Iwasa S, Yanai T, Hirano H, Shoji H, Honma Y, Okita N, Takashima A, Kato K, Hashimoto H, Sekiguchi M, Makino Y, Boku N, Yamaguchi M. Impact of peripheral neuropathy induced by platinum in first-line chemotherapy on second-line chemotherapy with paclitaxel for advanced gastric cancer. Int J Clin Oncol, 25:595-601, 2020
21. Satomi-Tsushita N, Honma Y, Nagashima K, Ito Y, Hirano H, Shoji H, Takashima A, Iwasa S, Kato K, Hamaguchi T, Itami J, Boku N. Risk Factors of Severe Benign Cicatricial Stricture After Definitive Chemoradiation for Localized T3 Esophageal Carcinoma. Anticancer Res, 40:1071-1077, 2020
22. Seo T, Noguchi E, Yoshida M, Mori T, Tanioka M, Sudo K, Shimomura A, Yonemori K, Fujiwara Y, Tamura K. Response to Dabrafenib and Trametinib of a Patient with Metaplastic Breast Carcinoma Harboring a BRAF V600E Mutation. Case Rep Oncol Med, 2020:2518383, 2020
23. Sudo K, Kato K, Matsuzaki J, Takizawa S, Aoki Y, Shoji H, Iwasa S, Honma Y, Takashima A, Sakamoto H, Naka T, Sekine S, Boku N, Ochiya T. Identification of serum microRNAs predicting the response of esophageal squamous-cell carcinoma to nivolumab. Jpn J Clin Oncol, 50:114-121, 2020
24. Tamura K, Imamura CK, Takano T, Saji S, Yamanaka T, Yonemori K, Takahashi M, Tsurutani J, Nishimura R, Sato K, Kitani A, Ueno NT, Mushiroda T, Kubo M, Fujiwara Y, Tanigawara Y. CYP2D6 Genotype-Guided Tamoxifen Dosing in Hormone Receptor-Positive Metastatic Breast Cancer (TARGET-1): A Randomized, Open-Label, Phase II Study. J Clin Oncol, 38:558-566, 2020
25. Tanabe Y, Shiraishi S, Hashimoto K, Ikeda K, Nishizawa D, Hasegawa J, Shimomura A, Ozaki Y, Tamura N, Yunokawa M, Yonemori K, Takano T, Kawabata H, Tamura K, Fujiwara Y, Shimizu C. Taxane-induced sensory peripheral neuropathy is associated with an SCN9A single nucleotide polymorphism in Japanese patients. BMC Cancer, 20:325, 2020
26. Yazaki S, Yamauchi T, Higashi T. High hepatitis B virus screening rate among patients receiving systemic anticancer treatment in Japan. Int J Clin Oncol, 25:1327-1333, 2020
27. Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S, Chen LT, Osada M, Helwig C, Dussault I, Ikeda M. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer, 8:e000564. doi: 10.1136/jitc-2020-000564, 2020
28. Okuno T, Arakawa S, Yoshida T, Ohe Y. Efficacy of osimertinib in a patient with leptomeningeal metastasis and EGFR uncommon S768I mutation. Lung Cancer, 143:95-96, 2020
29. Koyama T, Kondo S, Shimizu T, Fujiwara Y, Morizane C, Sakamoto Y, Okusaka T, Yamamoto N. Impact of Hepatitis Virus on the Feasibility and Efficacy of Anticancer Agents in Patients With Hepatocellular Carcinoma in Phase I Clinical Trials. Front Oncol, 9:301, 2019
30. Iizumi S, Kuchiba A, Okusaka T, Ikeda M, Sakamoto Y, Kondo S, Morizane C, Ueno H, Osame K, Mitsunaga S, Ohno I, Imaoka H, Hashimoto Y, Takahashi H, Sasaki M, Ohashi K. Impact of the Duration of Diabetes Mellitus on the Outcome of Metastatic Pancreatic Cancer Treated with Gemcitabine: A Retrospective Study. Intern Med, 58:2435-2441, 2019
31. Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, Okano N, Kimura K, Asada S, Namba Y, Okusaka T, Furuse J. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol, 4:611-621, 2019
32. Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, Koyama T, Kakishima H, Kitami M, Matsushita H, Furukawa E, Narushima D, Nagai M, Taniguchi H, Motoi N, Sekine S, Maeshima A, Mori T, Watanabe R, Yoshida M, Yoshida A, Yoshida H, Satomi K, Sukeda A, Hashimoto T, Shimizu T, Iwasa S, Yonemori K, Kato K, Morizane C, Ogawa C, Tanabe N, Sugano K, Hiraoka N, Tamura K, Yoshida T, Fujiwara Y, Ochiai A, Yamamoto N, Kohno T. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci, 110:1480-1490, 2019
33. Mizuguchi Y, Sakamoto T, Hashimoto T, Tsukamoto S, Iwasa S, Saito Y, Sekine S. Identification of a novel PRR15L-RSPO2 fusion transcript in a sigmoid colon cancer derived from superficially serrated adenoma. Virchows Arch, 475:659-663, 2019
34. Watanabe J, Shoji H, Hamaguchi T, Miyamoto T, Hirano H, Iwasa S, Honma Y, Takashima A, Kato K, Ito Y, Itami J, Kanemitsu Y, Boku N. Chemoradiotherapy for Local Recurrence of Rectal Cancer: A Single Center Study of 18 Patients. In Vivo, 33:1363-1368, 2019
35. Sudo K, Kato K, Matsuzaki J, Boku N, Abe S, Saito Y, Daiko H, Takizawa S, Aoki Y, Sakamoto H, Niida S, Takeshita F, Fukuda T, Ochiya T. Development and Validation of an Esophageal Squamous Cell Carcinoma Detection Model by Large-Scale MicroRNA Profiling. JAMA Netw Open, 2:e194573, 2019
36. Ando Y, Iwasa S, Takahashi S, Saka H, Kakizume T, Natsume K, Suenaga N, Quadt C, Yamada Y. Phase I study of alpelisib (BYL719), an α-specific PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci, 110:1021-1031, 2019
37. Aoki M, Shoji H, Nagashima K, Imazeki H, Miyamoto T, Hirano H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Higuchi K, Boku N. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open, 4:e000488, 2019
38. Arai H, Iwasa S, Boku N, Kawahira M, Yasui H, Masuishi T, Muro K, Minashi K, Hironaka S, Fukuda N, Takahari D, Nakajima TE. Fluoropyrimidine with or without platinum as first-line chemotherapy in patients with advanced gastric cancer and severe peritoneal metastasis: a multicenter retrospective study. BMC Cancer, 19:652, 2019
39. Doi T, Fujiwara Y, Matsubara N, Tomomatsu J, Iwasa S, Tanaka A, Endo-Tsukude C, Nakagawa S, Takahashi S. Phase I study of ipatasertib as a single agent and in combination with abiraterone plus prednisolone in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 84:393-404, 2019
40. Doi T, Iwasa S, Muro K, Satoh T, Hironaka S, Esaki T, Nishina T, Hara H, Machida N, Komatsu Y, Shimada Y, Otsu S, Shimizu S, Watanabe M. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial. Gastric Cancer, 22:817-827, 2019
41. Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, Oizumi S, Gemmoto K, Suna H, Enokitani K, Kawakami T, Nishikawa H, Yamamoto N. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin Cancer Res, 25:6614-6622, 2019
42. Goto K, Fujiwara Y, Isobe T, Chayahara N, Kiyota N, Mukohara T, Tsubata Y, Hotta T, Tamura K, Yamamoto N, Minami H. Pharmacokinetic study of the oral fluorouracil antitumor agent S-1 in patients with impaired renal function. Cancer Sci, 110:1987-1994, 2019
43. Hirakawa A, Sudo K, Yonemori K, Sadachi R, Kinoshita F, Kobayashi Y, Okuma HS, Kawachi A, Tamura K, Fujiwara Y, Rubinstein L, Takebe N. A Comparative Study of Longitudinal Toxicities of Cytotoxic Drugs, Molecularly Targeted Agents, Immunomodulatory Drugs, and Cancer Vaccines. Clin Pharmacol Ther, 106:803-809, 2019
44. Ida H, Honma Y, Hirano H, Shoji H, Iwasa S, Okita N, Takashima A, Kato K, Fukuda T, Boku N. Clinical outcomes of patients with G1/G2 neuroendocrine tumors arising from foregut or hindgut treated with somatostatin analogs: a retrospective study. Invest New Drugs, 37:573-578, 2019
45. Inagaki C, Shimoi T, Sumiyoshi Okuma H, Kawachi A, Sudo K, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Arakawa A, Ogawa C, Yoshida A, Fujiwara Y, Tamura K. Bone marrow examination in patients with Ewing sarcoma/peripheral primitive neuroectodermal tumor without metastasis based on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography. Med Oncol, 36:58, 2019
46. Ito T, Honma Y, Hirano H, Shoji H, Okita N, Iwasa S, Takashima A, Kato K, Boku N. S-1 Monotherapy After Failure of Platinum Plus 5-Fluorouracil Chemotherapy in Recurrent or Metastatic Esophageal Carcinoma. Anticancer Res, 39:3931-3936, 2019
47. Katsuya Y, Horinouchi H, Seto T, Umemura S, Hosomi Y, Satouchi M, Nishio M, Kozuki T, Hida T, Sukigara T, Nakamura K, Kuchiba A, Ohe Y. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer, 113:78-86, 2019
48. Kawajiri A, Kitano S, Maeshima AM, Inamoto Y, Tajima K, Takemura T, Tanaka T, Ito A, Okinaka K, Kurosawa S, Kim SW, Taniguchi H, Ogawa C, Izutsu K, Yamamoto N, Fukuda T. Association of CD204+ macrophages with poor outcomes of malignant lymphomas not in remission treated by allogeneic HCT. Eur J Haematol, 103:578-587, 2019
49. Li CK, Dalvi R, Yonemori K, Ariffin H, Lyu CJ, Farid M, Gonzales-Santos JRN, Zhou Q, Bielack S, Brugieres L, Blondeel A, Essiaf S, Peccatori FA, Jezdic S, Stark DP, Douillard JY, Saloustros E, Mountzios G. Care of adolescents and young adults with cancer in Asia: results of an ESMO/SIOPE/SIOP Asia survey. ESMO Open, 4:e000467, 2019
50. Loong HH, Tan DSW, Shimizu T. Challenges and insights of early phase oncology drug development in the Asia-Pacific region. Chin Clin Oncol, 8:26, 2019
51. Makino Y, Makihara-Ando R, Ogawa T, Sato H, Goto Y, Kanda S, Horinouchi H, Fujiwara Y, Ohe Y, Yamamoto N. Individual optimal dose of amrubicin to prevent severe neutropenia in Japanese patients with lung cancer. Cancer Sci, 110:3573-3583, 2019
52. Masuda K, Fujiwara Y, Shinno Y, Mizuno T, Sato J, Morita R, Matsumoto Y, Murakami S, Goto Y, Kanda S, Horinouchi H, Yamamoto N, Ohe Y. Efficacy and safety of crizotinib in patients with ROS1 rearranged non-small cell lung cancer: a retrospective analysis. J Thorac Dis, 11:2965-2972, 2019
53. Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H, Aoki M, Miyamoto T, Hirano H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Boku N. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer, 19:974, 2019
54. Monma S, Kato K, Shouji H, Okita N, Takashima A, Honma Y, Iwasa S, Hamaguchi T, Yamada Y, Shimada Y, Boku N, Nagashima K, Ito Y, Itami J. Gastric mucosal injury and hemorrhage after definitive chemoradiotherapy for locally advanced esophageal cancer. Esophagus, 16:402-407, 2019
55. Muto Y, Kitano S, Tsutsumida A, Namikawa K, Takahashi A, Nakamura Y, Yamanaka T, Yamamoto N, Yamazaki N. Investigation of clinical factors associated with longer overall survival in advanced melanoma patients treated with sequential ipilimumab. J Dermatol, 46:498-506, 2019
56. Nagata Y, Sawada R, Takashima A, Shoji H, Honma Y, Iwasa S, Amano K, Kato K, Hamaguchi T, Shimada Y, Saruta M, Boku N. Efficacy and safety of pemetrexed plus cisplatin as first-line chemotherapy in advanced malignant peritoneal mesothelioma. Jpn J Clin Oncol, 49:1004-1008, 2019
57. Naito T, Udagawa H, Sato J, Horinouchi H, Murakami S, Goto Y, Kanda S, Fujiwara Y, Yamamoto N, Zenke Y, Kirita K, Matsumoto S, Yoh K, Niho S, Motoi N, Ohe Y, Ishii G, Goto K. A Minimum Of 100 Tumor Cells in a Single Biopsy Sample Is Required to Assess Programmed Cell Death Ligand 1 Expression in Predicting Patient Response to Nivolumab Treatment in Nonsquamous Non-Small Cell Lung Carcinoma. J Thorac Oncol, 14:1818-1827, 2019
58. Noda-Narita S, Shimomura A, Kawachi A, Sumiyoshi-Okuma H, Sudo K, Shimoi T, Noguchi E, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Comparison of the efficacy of trastuzumab emtansine between patients with metastatic human epidermal growth factor receptor 2-positive breast cancers previously treated with combination trastuzumab and pertuzumab and with trastuzumab only in Japanese population. Breast Cancer, 26:492-498, 2019
59. Nokihara H, Nishio M, Yamamoto N, Fujiwara Y, Horinouchi H, Kanda S, Horiike A, Ohyanagi F, Yanagitani N, Nguyen L, Yaron Y, Borgman A, Tamura T. Phase 1 Study of Cabozantinib in Japanese Patients With Expansion Cohorts in Non-Small-Cell Lung Cancer. Clin Lung Cancer, 20:e317-e328, 2019
60. Sato J, Shimoi T, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Fujiwara Y, Yoshida M, Tamura K. The Incidence of Nonmalignant Diseases among Patients with Suspected Carcinoma of Unknown Primary Site. Intern Med, 58:1423-1428, 2019
61. Sato J, Shimomura A, Kawauchi J, Matsuzaki J, Yamamoto Y, Takizawa S, Sakamoto H, Ohno M, Narita Y, Ochiya T, Tamura K. Brain metastasis-related microRNAs in patients with advanced breast cancer. PLoS One, 14:e0221538, 2019
62. Shibaki R, Murakami S, Matsumoto Y, Goto Y, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N, Motoi N, Kusumoto M, Yamamoto N, Ohe Y. Tumor expression and usefulness as a biomarker of programmed death ligand 1 in advanced non-small cell lung cancer patients with preexisting interstitial lung disease. Med Oncol, 36:49, 2019
63. Shibaki R, Murakami S, Shinno Y, Matsumoto Y, Goto Y, Kanda S, Horinouchi H, Fujiwara Y, Motoi N, Yamamoto N, Ohe Y. Malignant pleural effusion as a predictor of the efficacy of anti-PD-1 antibody in patients with non-small cell lung cancer. Thorac Cancer, 10:815-822, 2019
64. Shimomura A, Yonemori K, Yoshida M, Yoshida T, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hamada A, Michimae H, Hashimoto J, Yamamoto H, Kawachi A, Shimizu C, Fujiwara Y, Tamura K. Gene Alterations in Triple-Negative Breast Cancer Patients in a Phase I/II Study of Eribulin and Olaparib Combination Therapy. Transl Oncol, 12:1386-1394, 2019
65. Shinno Y, Goto Y, Sato J, Morita R, Matsumoto Y, Murakami S, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N, Ohe Y. Mixed response to osimertinib and the beneficial effects of additional local therapy. Thorac Cancer, 10:738-743, 2019
66. Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, Tsurutani J, Kadowaki S, Yamaguchi K, Iwasa S, Saito K, Fujisaki Y, Sugihara M, Shahidi J, Doi T. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol, 20:827-836, 2019
67. Shitara K, Satoh T, Iwasa S, Yamaguchi K, Muro K, Komatsu Y, Nishina T, Esaki T, Hasegawa J, Kakurai Y, Kamiyama E, Nakata T, Nakamura K, Sakaki H, Hyodo I. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: a first-in-human phase I dose escalation and dose expansion study in patients with advanced solid tumors. J Immunother Cancer, 7:219, 2019
68. Sone M, Arai Y, Sugawara S, Kubo T, Itou C, Hasegawa T, Umakoshi N, Yamamoto N, Sunami K, Hiraoka N, Kubo T. Feasibility of genomic profiling with next-generation sequencing using specimens obtained by image-guided percutaneous needle biopsy. Ups J Med Sci, 124:119-124, 2019
69. Tamura N, Horinouchi H, Sekine K, Matsumoto Y, Murakami S, Goto Y, Kanda S, Fujiwara Y, Yamamoto N, Ohe Y. Efficacy of subsequent docetaxel +/- ramucirumab and S-1 after nivolumab for patients with advanced non-small cell lung cancer. Thorac Cancer, 10:1141-1148, 2019
70. Toki S, Kobayashi E, Yoshida A, Ogura K, Wakai S, Yoshimoto S, Yonemori K, Kawai A. A clinical comparison between dedifferentiated low-grade osteosarcoma and conventional osteosarcoma. Bone Joint J, 101-B:745-752, 2019
71. Watanabe S, Honma Y, Murakami N, Igaki H, Mori T, Hirano H, Okita N, Shoji H, Iwasa S, Takashima A, Kato K, Kobayashi K, Matsumoto F, Yoshimoto S, Itami J, Boku N. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy for unresectable sinonasal undifferentiated carcinoma: Two cases of report. World J Clin Cases, 7:765-772, 2019
72. Yamada Y, Boku N, Mizusawa J, Iwasa S, Kadowaki S, Nakayama N, Azuma M, Sakamoto T, Shitara K, Tamura T, Chin K, Hata H, Nakamori M, Hara H, Yasui H, Katayama H, Fukuda H, Yoshikawa T, Sasako M, Terashima M. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol, 4:501-510, 2019
73. Yamaguchi T, Iwasa S, Shoji H, Honma Y, Takashima A, Kato K, Hamaguchi T, Higuchi K, Boku N. Association between UGT1A1 gene polymorphism and safety and efficacy of irinotecan monotherapy as the third-line treatment for advanced gastric cancer. Gastric Cancer, 22:778-784, 2019
74. Yonemori K, Shimomura A, Yasojima H, Masuda N, Aogi K, Takahashi M, Naito Y, Shimizu S, Nakamura R, Hashimoto J, Yamamoto H, Hirakawa A, Michimae H, Hamada A, Yoshida T, Sukigara T, Tamura K, Fujiwara Y. A phase I/II trial of olaparib tablet in combination with eribulin in Japanese patients with advanced or metastatic triple-negative breast cancer previously treated with anthracyclines and taxanes. Eur J Cancer, 109:84-91, 2019
75. Yoshida K, Kanda S, Shiraishi H, Goto K, Itahashi K, Goto Y, Horinouchi H, Fujiwara Y, Nokihara H, Yamamoto N, Ohe Y. Difference in central nerve system metastasis during gefitinib or erlotinib therapy in patients with EGFR-mutated non-small cell lung cancer: a retrospective study. J Thorac Dis, 11:1347-1354, 2019
76. Yunokawa M, Sasada S, Takehara Y, Takahashi K, Shimoi T, Yonemori K, Ishikawa M, Kato T, Tamura K. Real-world data on initial treatment strategies for older adult patients with endometrial cancer in Japan. Cancer Chemother Pharmacol, 84:1051-1058, 2019
77. Kuroda T, Ogiwara H, Sasaki M, Takahashi K, Yoshida H, Kiyokawa T, Sudo K, Tamura K, Kato T, Okamoto A, Kohno T. Therapeutic preferability of gemcitabine for ARID1A-deficient ovarian clear cell carcinoma. Gynecol Oncol, 155:489-498, 2019
