Annual Report 2020
Department of Head and Neck Medical Oncology
Makoto Tahara, Susumu Okano, Yuri Ueda, Takao Fujisawa, Akihisa Wada, Hideki Tanaka, Masanobu Sato
Introduction
The Department of Head and Neck Medical Oncology is engaged in the clinical management of patients with head and neck cancer (HNC), and research into anticancer drugs for the treatment of HNC.
Our missions are to: 1) provide the best evidence-based treatment; 2) promote the importance of supportive care in the treatment of patients with HNC; 3) facilitate the timely approval of new drugs by active participation in global clinical trials to eliminate the drug lag; 4) develop cutting-edge treatments; and 5) train experts in head and neck medical oncology.
The Team and What We Do
Our department consists of three physicians, one senior resident and one resident. We manage the treatment of HNC patients who receive anticancer drugs. An estimated 60% of HNC patients require a multidisciplinary approach, including surgery, radiotherapy, and chemotherapy. Given the increasing complexity of the management of HNC, recommended treatment for patients who are referred to our institution is decided at weekly tumor board attended by a multidisciplinary team.
A total of 345 patients were referred to our department from April 2020 to March 2021 (Table 1, Table 2). The outpatient service of our department is available from Monday to Friday. We carefully follow patients during and after treatment and provide palliative chemotherapy as an outpatient service.
Table 1. Number of patients by site

Table 2. Number of patients by procedure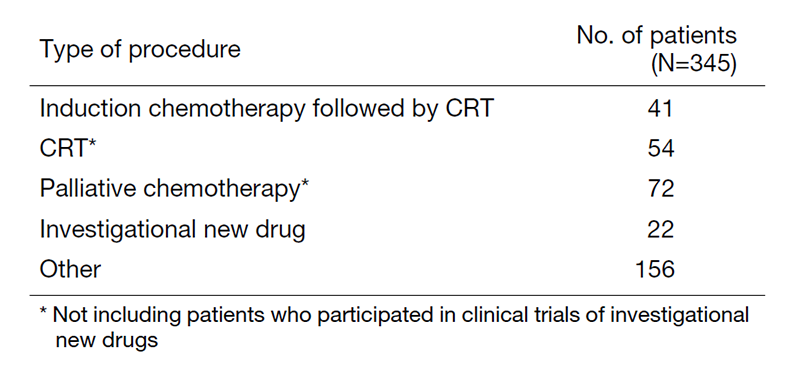

Research activities
Our research activities have focused on three areas: the development of new treatments in clinical trials for HNC, biomarker analysis in HNC and retrospective analysis of management of the treatment for HNC.
Salivary gland carcinoma (SGC) is a rare malignant tumor that accounts for 8% of all head and neck cancers. Although a variety of chemotherapies and molecular-targeted therapies have been tested as systemic treatments for SGC, the standard regimen has not yet been established. Androgen receptor (AR) expression is observed in some cases of SGC, especially in salivary duct carcinoma (SDC). In several studies, hormone therapy targeting AR demonstrated promising clinical activity against AR-positive SDC. We conducted a phase 2 study of darolutamide for patients with AR-positive SDC. Patient enrollment was completed and a total of 24 patients received study drug. Recently, a combined androgen blockade (CAB) with androgen blockade and luteinizing hormone–releasing hormone (LH-RH) agonists demonstrated promising activity in a prospective study. Therefore, we are now planning to conduct a CAB cohort with darolutamide and LH-RH agonists.
Clinical trials
The following investigator-initiated clinical trials are ongoing: 1) a phase 2 study of lenvatinib for anaplastic thyroid cancer, 2) a cohort study exploring the effect of lenvatinib on differentiated thyroid cancer, 3) a phase 2 study of combination with nivolumab plus lenvatinib for unresectable anaplastic thyroid cancer and 4) a phase 2 study of darolutamide for androgen receptor positive recurrent or metastatic salivary gland carcinoma.
To facilitate the timely approval of new drugs and eliminate the drug lag, we have also participated in the global phase trials including immune-checkpoint inhibitors.
Education
We educate not only medical staff in our institute but also outside our institute by conducting the following education program: Seminar of the Japan Society of Supportive Care for patients with HNC. Furthermore, our department is accepting trainees all the time.
Future Prospects
We hope that ongoing or planned clinical trials will change the standard of care for HNC and our biomarker analysis will lead to the development of new treatment strategies. Our education program will increase the number of medical oncologists who take charge of treatment for HNC, leading to improving patient’s quality of survival.
List of papers published in 2020
Journal
1. Shiga K, Nibu KI, Fujimoto Y, Asakage T, Homma A, Mitani H, Ogawa T, Okami K, Murono S, Hirano S, Ueda T, Hanai N, Tsukahara K, Ota I, Yoshimoto S, Shinozaki T, Iwae S, Katagiri K, Saito D, Kiyota N, Tahara M, Takahashi F, Hayashi R. Multi-institutional Survey of Squamous Cell Carcinoma of the External Auditory Canal in Japan. Laryngoscope, 131:E870-E874, 2021
2. Okano S, Homma A, Kiyota N, Tahara M, Hanai N, Asakage T, Matsuura K, Ogawa T, Saito Y, Sano D, Kodaira T, Motegi A, Yasuda K, Takahashi S, Tanaka K, Onoe T, Yokota T, Imamura Y, Ariizumi Y, Akimoto T, Hayashi R. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck. Jpn J Clin Oncol, 51:173-179, 2021
3. Adkins DR, Lin JC, Sacco A, Ley J, Oppelt P, Vanchenko V, Komashko N, Yen CJ, Wise-Draper T, Lopez-Picazo Gonzalez J, Radulovic S, Shen Q, Thurm H, Martini JF, Hoffman J, Huang X, Melichar B, Tahara M. Palbociclib and cetuximab compared with placebo and cetuximab in platinum-resistant, cetuximab-naïve, human papillomavirus-unrelated recurrent or metastatic head and neck squamous cell carcinoma: A double-blind, randomized, phase 2 trial. Oral Oncol, 115:105192, 2021
4. Enokida T, Tahara M. Electrochemotherapy in the Treatment of Head and Neck Cancer: Current Conditions and Future Directions. Cancers (Basel), 13:2021
5. Katada C, Sugawara M, Hara H, Fujii H, Nakajima TE, Ando T, Kojima T, Watanabe A, Sakamoto Y, Ishikawa H, Hosokawa A, Hamamoto Y, Muto M, Tahara M, Koizumi W. A management of neutropenia using granulocyte colony stimulating factor support for chemotherapy consisted of docetaxel, cisplatin and 5-fluorouracil in patients with oesophageal squamous cell carcinoma. Jpn J Clin Oncol, 51:199-204, 2021
6. Tahara M, Kiyota N, Hoff AO, Badiu C, Owonikoko TK, Dutcus CE, Suzuki T, Ren M, Wirth LJ. Impact of lung metastases on overall survival in the phase 3 SELECT study of lenvatinib in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer, 147:51-57, 2021
7. Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, Harrington K, Chang PM, Lin JC, Razaq MA, Teixeira MM, Lövey J, Chamois J, Rueda A, Hu C, Dunn LA, Dvorkin MV, De Beukelaer S, Pavlov D, Thurm H, Cohen E. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol, 22:450-462, 2021
8. Kramer R, Zaremba A, Moreira A, Ugurel S, Johnson DB, Hassel JC, Salzmann M, Gesierich A, Weppler A, Spain L, Loquai C, Dudda M, Pföhler C, Hepner A, Long GV, Menzies AM, Carlino MS, Sachse MM, Lebbé C, Baroudjian B, Enokida T, Tahara M, Schlaak M, Hayani K, Bröckelmann PJ, Meier F, Reinhardt L, Friedlander P, Eigentler T, Kähler KC, Berking C, Zimmer L, Heinzerling L. Hematological immune related adverse events after treatment with immune checkpoint inhibitors. Eur J Cancer, 147:170-181, 2021
9. Tsushima N, Shinozaki T, Fujisawa T, Tomioka T, Okano W, Ikeda M, Tahara M, Higashino T, Hayashi R. Salvage Reconstructive Surgery During Nivolumab Therapy for a Patient With Hypopharyngeal Cancer. Clin Med Insights Case Rep, 13:1179547620908854, 2020
10. Takahashi S, Tahara M, Ito K, Tori M, Kiyota N, Yoshida K, Sakata Y, Yoshida A. Safety and Effectiveness of Lenvatinib in 594 Patients with Unresectable Thyroid Cancer in an All-Case Post-Marketing Observational Study in Japan. Adv Ther, 37:3850-3862, 2020
11. Suzuki S, Horinouchi A, Uozumi S, Matsuyama C, Kamata H, Kaneko A, Yamaguchi M, Okudera H, Tahara M, Kawasaki T. Impact of outpatient pharmacy interventions on management of thyroid patients receiving lenvatinib. SAGE Open Med, 8:2050312120930906, 2020
12. Okumura M, Motegi A, Zenda S, Nakamura N, Hojo H, Nakamura M, Hirano Y, Kageyama SI, Arahira S, Parshuram RV, Kuno H, Hayashi R, Tahara M, Itoh Y, Naganawa S, Akimoto T. Efficacy and safety of accelerated fractionated radiotherapy without elective nodal irradiation for T3N0 glottic cancer without vocal cord fixation. Head Neck, 42:1775-1782, 2020
13. Mehanna H, Rischin D, Wong SJ, Gregoire V, Ferris R, Waldron J, Le QT, Forster M, Gillison M, Laskar S, Tahara M, Psyrri A, Vermorken J, Porceddu S. De-Escalation After DE-ESCALATE and RTOG 1016: A Head and Neck Cancer InterGroup Framework for Future De-Escalation Studies. J Clin Oncol, 38:2552-2557, 2020
14. Maruo T, Zenda S, Shinozaki T, Tomioka T, Okano W, Sakuraba M, Tahara M, Hayashi R. Comparison of salvage surgery for recurrent or residual head and neck squamous cell carcinoma. Jpn J Clin Oncol, 50:288-295, 2020
15. Machiels JP, Tao Y, Burtness B, Tahara M, Licitra L, Rischin D, Waldron J, Simon C, Gregoire V, Harrington K, Alves GV, Figueiredo Lima IP, Pointreau Y, M Hughes BG, Aksoy S, Hetnal M, Ge JY, Brown H, Cheng J, Bidadi B, Siu LL. Pembrolizumab given concomitantly with chemoradiation and as maintenance therapy for locally advanced head and neck squamous cell carcinoma: KEYNOTE-412. Future Oncol, 16:1235-1243, 2020
16. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N, Mascarenhas L, Geoerger B, Dowlati A, Pappo AS, Bielack S, Doz F, McDermott R, Patel JD, Schilder RJ, Tahara M, Pfister SM, Witt O, Ladanyi M, Rudzinski ER, Nanda S, Childs BH, Laetsch TW, Hyman DM, Drilon A. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol, 21:531-540, 2020
17. Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, Clement PM, Mesia R, Kutukova S, Zholudeva L, Daste A, Caballero-Daroqui J, Keam B, Vynnychenko I, Lafond C, Shetty J, Mann H, Fan J, Wildsmith S, Morsli N, Fayette J, Licitra L. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol, 31:942-950, 2020
18. Ueda Y, Enokida T, Okano S, Fujisawa T, Ito K, Tahara M. Combination Treatment With Paclitaxel, Carboplatin, and Cetuximab (PCE) as First-Line Treatment in Patients With Recurrent and/or Metastatic Nasopharyngeal Carcinoma. Front Oncol, 10:571304, 2020
19. Enokida T, Ogawa T, Homma A, Okami K, Minami S, Nakanome A, Shimizu Y, Maki D, Ueda Y, Fujisawa T, Motegi A, Ohkoshi A, Taguchi J, Ebisumoto K, Nomura S, Okano S, Tahara M. A multicenter phase II trial of paclitaxel, carboplatin, and cetuximab followed by chemoradiotherapy in patients with unresectable locally advanced squamous cell carcinoma of the head and neck. Cancer Med, 9:1671-1682, 2020
20. Watanabe R, Okano S, Yamazaki N. Fixed drug eruption dramatically exacerbated during treatment with programmed death 1 inhibitor. J Dermatol, 47:e425-e426, 2020
21. Yokota T, Homma A, Kiyota N, Tahara M, Hanai N, Asakage T, Matsuura K, Ogawa T, Saito Y, Sano D, Kodaira T, Motegi A, Yasuda K, Takahashi S, Tanaka K, Onoe T, Okano S, Imamura Y, Ariizumi Y, Hayashi R. Immunotherapy for squamous cell carcinoma of the head and neck. Jpn J Clin Oncol, 50:1089-1096, 2020
22. Kunieda F, Kiyota N, Tahara M, Kodaira T, Hayashi R, Ishikura S, Mizusawa J, Nakamura K, Fukuda H, Fujii M. Randomized phase II/III trial of post-operative chemoradiotherapy comparing 3-weekly cisplatin with weekly cisplatin in high-risk patients with squamous cell carcinoma of head and neck: Japan Clinical Oncology Group Study (JCOG1008). Jpn J Clin Oncol, 44:770-774, 2014
