Annual Report 2020
Department of Thoracic Oncology
Koichi Goto, Kiyotaka Yoh, Shingo Matsumoto, Yoshitaka Zenke, Hibiki Udagawa, Takaya Ikeda, Kaname Nosaki, Hiroki Izumi, Eri Sugiyama, Yuji Shibata, Tetsuya Sakai, Akira Sugimoto, Yutaro Tamiya, Masanobu Okahisa, Yosuke Kagawa, Yu Tanaka, Hajime Oi, Fumihiko Okumura, Shingo Kitagawa, Tatsunori Kiriu, Shunta Mori, Mao Sasamoto, (Shigeki Umemura, Seiji Niho, Keisuke Kirita)
Introduction
The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. The division aims to provide the highest quality treatment and establish new effective treatments against lung cancer and other thoracic malignancies through innovative clinical and translational research. To provide assistance to our patients through multidisciplinary care, the staff members of the division work closely with thoracic surgeons, radiation oncologists, pathologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.
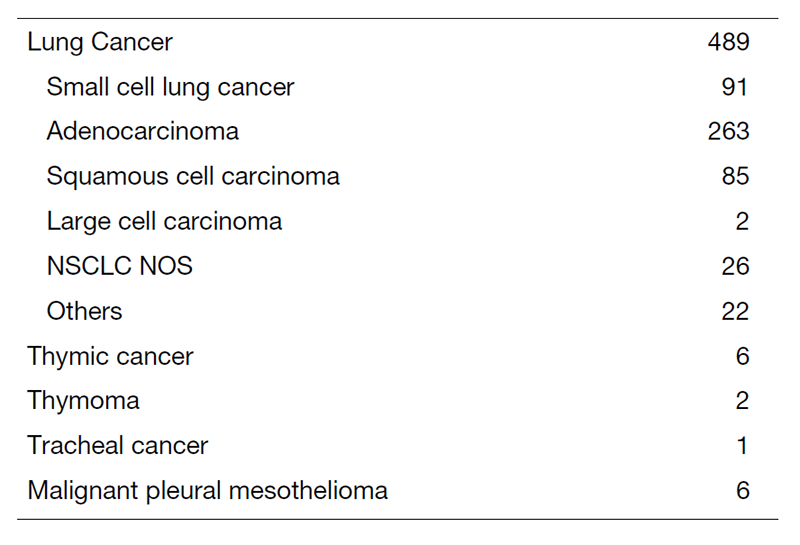
The Team and What We Do
Our Outpatient Clinic, managed by the staff members and senior residents, is open from Monday to Friday for the examination of all new referred patients and the evaluation of returning patients. Returning patients also receive oral chemotherapy and/or intravenous chemotherapy in the Ambulatory Care Center. Bronchoscopy with EBUS for diagnosis is performed from Monday to Thursday afternoon. Fluoroscopic-CT guided needle lung biopsies are carried out on Tuesday afternoon. For patient management, we use approximately 60 beds in mainly 8F, 6A, 6B and 5A wards.
Case conferences on thoracic surgery and medical oncology are scheduled on Tuesday evenings and Wednesday evenings, respectively. The staff members and residents of the division participate in a journal club on Monday and Wednesday mornings. At monthly meetings with physicians in private hospitals, the staff members and residents are teaching methods of reading for chest X-ray and CT images.
Table 2. Type of First-Line Treatment

Research activities
Our research activities are focused on three areas: 1) development of new and effective diagnosis and treatment modalities in lung cancer; 2) collaborative studies with the Basic Research Center for Innovative Oncology in the following areas: detection of biomarkers for the treatment of advanced lung cancer; development of new diagnostic methods for lung cancer With rare driver genomic alteration; correlation between genomic abnormalities and clinical characteristics and treatment in lung cancer; correlation between pathological features and sensitivity of treatments in lung cancer; and 3) translational research from bench to bed-side or from bed-side to bench for the development of innovative treatment strategies in lung cancer.
Especially, high-spec genomic analysis technologies are adapted to our research screening for rare driver genomic alterations of lung cancer such as RET, ROS1, BRAF, MET, and HER2 etc., and its clinical development is also supported collaborating with some diagnostic companies.
Clinical trials
The Department of Thoracic Oncology is currently conducting and participating in multi-institutional clinical studies for advanced lung cancer disease, such as the Japan Clinical Oncology Group (JCOG) trials, West Japan Oncology Group (WJOG) trials, Thoracic Oncology Research Group (TORG) trials, investigator initiated trials, and pharmaceutical company initiated global trials.
An Asian international genomic screening platform established by our department, whose name is LC-SCRUM-Asia, was initiated in 2013 and is now ongoing. As of March 2021, 187 Japanese institutions participated in LC-SCRUM-Asia and 12,528 patients were enrolled. In addition, 6 institutions in Taiwan participated in our genomic screening from 2019 March and 253 patients were already enrolled from Taiwan as February 2021. LC-SCRUM-Asia will support the development of novel therapeutic and diagnostic products in Asia and contribute to establish precision medicine in Asian countries. A lot of lung cancers with rare driver oncogenes, such as RET, ROS1 BRAF, MET, HER2 and KRAS genomic alterations were identified in our screening and they were entered into various clinical trials of molecular targeting agents. Based on the results of clinical trials leveraging genomic screening in LC-SCRUM-Asia, crizotinib was approved for ROS1 fusion positive lung cancer in May 2017, dabrafenib/trametinib was approved for BRAF V600E positive lung cancer in March 2018, entrectinib was approved for NTRK and ROS1 fusion positive lung cancer in June 2019 and February 2020, respectively, and tepotinib and capmatinib were approved for MET ex14 skipping positive lung cancer in March 2020 and June 2020, respectively. While RT-PCR kit which was adapted in LC-SCRUM-Asia screening for ROS1 fusion was simultaneously approved using our screening data as a companion diagnostic (CDx) for ROS1 positive lung cancer in January 2017. In the same way, next generation sequencing (NGS) panel was first approved as a CDx for BRAF mutation positive lung cancer in April 2018 and also approved as a first multi NGS CDx for EGFR/ALK/ROS1/BRAF in February 2019. Through the genomic screening and the clinical-genomic database established, LC-SCRUM-Asia should play a key role to develop precision medicine in lung cancer in Japan and Asian countries.
To confirm the usefulness of NGS panel using plasma cell-free DNA (liquid biopsy system), we also started LC-SCRUM-Liquid from December 2017 targeting 2000 advanced lung cancers and it is now ongoing. As of February 2021, 1143 patients were already enrolled into LC-SCRUM-Liquid and a concordance study between tissue and liquid NGS analyses is ongoing to evaluate the sensitivity of liquid biopsy system. We simultaneously initiated the umbrella-type clinical trials of osimertinib for EGFR mutation positive lung cancer by liquid biopsy from September 2019 and alectinib for ALK fusion positive lung cancer by liquid biopsy from October 2019. We would like to conduct large umbrella trials targeting various type of driver alterations based on our liquid screening.
To select optimal treatment for the individual patients with advanced lung cancer, we currently need to analyze many biomarkers including EGFR ALK, ROS1, BRAF, MET and NTRK genomic alterations by NGS analysis and PD-L1 immunohistochemical staining using tissue samples. Since we need to get large amount of tissue samples as possible by bronchoscopy for the biomarker analyses, we are conducting a feasibility study of cutting-edge trans-bronchial biopsy technique to evaluate its utility.
Education
Residents, cancer specialists in training, and staff are paired to provide outpatient/inpatient medical care and perform examinations. The aim is to develop clinicians who can provide comprehensive medical care for patients with thoracic malignant tumors, from diagnosis to treatment, and including palliative care, by closely supporting patients with advanced lung cancer for whom a complete cure is difficult. Moreover, in our department, we always try to train specialists who have not only outstanding minds but also strong mental and physical strengths to handle patient’s distress. Residents are required to go on rotation in the Department of Pathology during their training period, and have opportunities to come into contact with basic research from this department. Furthermore, we also actively support the preparation of manuscripts on basic and clinical research, aiming to develop clinicians capable of conducting clinical and translational research. In addition, two residents are affiliated with the Joint Graduate Program at Juntendo University, and two specialized researchers have been studying at Georgetown University in the United States since September 2018 and MD Anderson Cancer Center in the United States since October 2020, respectively.
A joint case conference with the Department of Thoracic Surgery is held every Tuesday and a joint case conference with the Department of Radiation Oncology is held every Wednesday to determine treatment policies, and a research conference is held once a month to discuss the progress and schedule of research with all members. A journal club with the Department of Thoracic Oncology is held every Monday, a journal club with the Department of Thoracic Surgery is held every Wednesday, a joint conference with the Departments of Thoracic Surgery and Pathology is held every Friday, and a chest X-ray reading meeting for local clinicians is held on the second Tuesday of each month, and a chest X-ray reading meeting with the Katsushika Medical Association is held on the fourth Tuesday of each month.
Future Prospects
Drug therapy for lung cancer has made a major shift to personalized medicine in which therapeutic drugs are selected based on biomarkers in individual patients. In addition, immune checkpoint inhibitors have greatly contributed to the improvement of treatment outcomes in lung cancer as highly effective drugs with a different mechanism of action from conventional drugs. Dramatic advances in these new therapies have greatly changed the treatment structure for advanced lung cancer in the last few years, and treatment outcomes have also significantly improved. In our department, we plan to continue conducting various research studies in the future with the aim of establishing personalized medicine for lung cancer based on biomarkers. Additionally, Asian countries with Thailand, Malaysia, Viet Nam and Singapore are taking part in LC-SCRUM-Asia, and in collaboration with LC-IRICA, a genomic screening platform in China, a large-scale international genomic screening platform in Asian countries will be established. By utilizing this screening platform, treatment development for advanced lung cancer, which has attracted a great deal of attention worldwide, will be conducted and the results will be released to the world. Moreover, we will continue to promote translational research, conduct innovative and advanced clinical trials that can link the results to clinical development, and pursue research with the aim of overcoming advanced lung cancer.
List of papers published in 2020
Journal
1. Nishio M, Yoshida T, Kumagai T, Hida T, Toyozawa R, Shimokawaji T, Goto K, Nakagawa K, Ohe Y, Seto T, Kudou K, Asato T, Zhang P, Yamamoto N. Brigatinib in Japanese Patients With ALK-Positive NSCLC Previously Treated With Alectinib and Other Tyrosine Kinase Inhibitors: Outcomes of the Phase 2 J-ALTA Trial. J Thorac Oncol, 16:452-463, 2021
2. Tanimoto A, Matsumoto S, Takeuchi S, Arai S, Fukuda K, Nishiyama A, Yoh K, Ikeda T, Furuya N, Nishino K, Ohe Y, Goto K, Yano S. Proteasome Inhibition Overcomes ALK-TKI Resistance in ALK-Rearranged/TP53-Mutant NSCLC via Noxa Expression. Clin Cancer Res, 27:1410-1420, 2021
3. Saka H, Nishio M, Hida T, Nakagawa K, Sakai H, Nogami N, Atagi S, Takahashi T, Horinouchi H, Takenoyama M, Katakami N, Tanaka H, Takeda K, Satouchi M, Isobe H, Maemondo M, Goto K, Hirashima T, Minato K, Yada N, Tamura T. Five-year follow-up results from phase II studies of nivolumab in Japanese patients with previously treated advanced non-small cell lung cancer: pooled analysis of the ONO-4538-05 and ONO-4538-06 studies. Jpn J Clin Oncol, 51:106-113, 2021
4. Amemiya R, Miyoshi T, Aokage K, Suzuki J, Hoshino H, Udagawa H, Tane K, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Goto K, Ikeda N, Tsuboi M, Ishii G. Prognostic impact of the tumor immune microenvironment in pulmonary pleomorphic carcinoma. Lung Cancer, 153:56-65, 2021
5. Sakai K, Tsuboi M, Kenmotsu H, Yamanaka T, Takahashi T, Goto K, Daga H, Ohira T, Ueno T, Aoki T, Nakagawa K, Yamazaki K, Hosomi Y, Kawaguchi K, Okumura N, Takiguchi Y, Sekine A, Haruki T, Yamamoto H, Sato Y, Akamatsu H, Seto T, Saeki S, Sugio K, Nishio M, Okabe K, Yamamoto N, Nishio K. Tumor mutation burden as a biomarker for lung cancer patients treated with pemetrexed and cisplatin (the JIPANG-TR). Cancer Sci, 112:388-396, 2021
6. Paty J, Sandin R, Reisman A, Wu YL, Migliorino MR, Zhou X, Cheng Y, Lee KH, Nakagawa K, Niho S, Corral J, Płużański A, Linke R, Meyers O, Mok TS. The patient's perspective on treatment with dacomitinib: patient-reported outcomes from the Phase III trial ARCHER 1050. Future Oncol, 17:783-794, 2021
7. Perets R, Bar J, Rasco DW, Ahn MJ, Yoh K, Kim DW, Nagrial A, Satouchi M, Lee DH, Spigel DR, Kotasek D, Gutierrez M, Niu J, Siddiqi S, Li X, Cyrus J, Chackerian A, Chain A, Altura RA, Cho BC. Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann Oncol, 32:395-403, 2021
8. Solomon BJ, Zhou CC, Drilon A, Park K, Wolf J, Elamin Y, Davis HM, Soldatenkova V, Sashegyi A, Lin AB, Lin BK, F Loong HH, Novello S, Arriola E, Perol M, Goto K, Santini FC. Phase III study of selpercatinib versus chemotherapy ± pembrolizumab in untreated RET positive non-small-cell lung cancer. Future Oncol, 17:763-773, 2021
9. Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Chawla A, Rosell R, Corral J, Migliorino MR, Pluzanski A, Noonan K, Tang Y, Pastel M, Wilner KD, Wu YL. Updated Overall Survival in a Randomized Study Comparing Dacomitinib with Gefitinib as First-Line Treatment in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. Drugs, 81:257-266, 2021
10. Shibata Y, Matsumoto S, Yoh K, Goto K. Evaluation of variant frequency in SARS-CoV-2 infection-related genes utilizing lung cancer genomic database. Lung Cancer, 152:199-201, 2021
11. Tamiya M, Kunimasa K, Nishino K, Matsumoto S, Kawachi H, Kuno K, Inoue T, Kuhara H, Imamura F, Goto K, Kumagai T. Successful treatment of an osimertinib-resistant lung adenocarcinoma with an exon 18 EGFR mutation (G719S) with afatinib plus bevacizumab. Invest New Drugs, 39:232-236, 2021
12. Arima H, Goto K, Motozawa T, Mouri M, Watanabe R, Hirano T, Ishikawa SE. Open-label, multicenter, dose-titration study to determine the efficacy and safety of tolvaptan in Japanese patients with hyponatremia secondary to syndrome of inappropriate secretion of antidiuretic hormone. Endocr J, 68:17-29, 2021
13. Furuya N, Matsumoto S, Kakinuma K, Morikawa K, Inoue T, Saji H, Goto K, Mineshita M. Suitability of transbronchial brushing cytology specimens for next-generation sequencing in peripheral lung cancer. Cancer Sci, 112:380-387, 2021
14. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Garassino MC, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Każarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Thiyagarajah P, Jiang H, Paz-Ares L. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol, 22:51-65, 2021
15. Takeuchi S, Yanagitani N, Seto T, Hattori Y, Ohashi K, Morise M, Matsumoto S, Yoh K, Goto K, Nishio M, Takahara S, Kawakami T, Imai Y, Yoshimura K, Tanimoto A, Nishiyama A, Murayama T, Yano S. Phase 1/2 study of alectinib in RET-rearranged previously-treated non-small cell lung cancer (ALL-RET). Transl Lung Cancer Res, 10:314-325, 2021
16. Yamamoto N, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Yamanaka T, Tanaka M, Takahashi K, Fukuoka M.Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: Survival follow-up results of the randomized JO25567 study. Lung Cancer, 151, 20-24, 2021.
17. Kenmotsu H, Niho S, Tsuboi M, Wakabayashi M, Ishii G, Nakagawa K, Daga H, Tanaka H, Saito H, Aokage K, Takahashi T, Menju T, Kasai T, Yoshino I, Minato K, Okada M, Eba J, Asamura H, Ohe Y, Watanabe SI. Randomized Phase III Study of Irinotecan Plus Cisplatin Versus Etoposide Plus Cisplatin for Completely Resected High-Grade Neuroendocrine Carcinoma of the Lung: JCOG1205/1206. J Clin Oncol, 38:4292-4301, 2020
18. Satouchi M, Nosaki K, Takahashi T, Nakagawa K, Aoe K, Kurata T, Sekine A, Horiike A, Fukuhara T, Sugawara S, Umemura S, Saka H, Okamoto I, Yamamoto N, Sakai H, Kishi K, Katakami N, Horinouchi H, Hida T, Okamoto H, Atagi S, Ohira T, Han SR, Noguchi K, Ebiana V, Hotta K. First-line pembrolizumab vs chemotherapy in metastatic non-small-cell lung cancer: KEYNOTE-024 Japan subset. Cancer Sci, 111:4480-4489, 2020
19. Fujisawa D, Umemura S, Okizaki A, Satomi E, Yamaguchi T, Miyaji T, Mashiko T, Kobayashi N, Kinoshita H, Mori M, Morita T, Uchitomi Y, Goto K, Ohe Y, Matsumoto Y. Nurse-led, screening-triggered, early specialised palliative care intervention programme for patients with advanced lung cancer: study protocol for a multicentre randomised controlled trial. BMJ Open, 10:e037759, 2020
20. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, McCoach CE, Gautschi O, Besse B, Cho BC, Peled N, Weiss J, Kim YJ, Ohe Y, Nishio M, Park K, Patel J, Seto T, Sakamoto T, Rosen E, Shah MH, Barlesi F, Cassier PA, Bazhenova L, De Braud F, Garralda E, Velcheti V, Satouchi M, Ohashi K, Pennell NA, Reckamp KL, Dy GK, Wolf J, Solomon B, Falchook G, Ebata K, Nguyen M, Nair B, Zhu EY, Yang L, Huang X, Olek E, Rothenberg SM, Goto K, Subbiah V. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med, 383:813-824, 2020
21. Zenke Y, Niho S, Umemura S, Ishihara M, Seki N, Nogami N, Hosomi Y, Shimokawa T, Tokito T, Goto Y, Miura Y, Saito H, Hida N, Ikeda S, Tanaka H, Furuya N, Misumi T, Yamanaka T, Ohe Y, Okamoto H. Phase I/II study of carboplatin plus weekly nab-paclitaxel in patients aged ≧75 years with squamous-cell lung cancer: TORG1322. Lung Cancer, 146:182-188, 2020
22. Seto T, Hayashi H, Satouchi M, Goto Y, Niho S, Nogami N, Hida T, Takahashi T, Sakakibara-Konishi J, Morise M, Nagasawa T, Suzuki M, Ohkura M, Fukuhara K, Thurm H, Peltz G, Nishio M. Lorlatinib in previously treated anaplastic lymphoma kinase-rearranged non-small cell lung cancer: Japanese subgroup analysis of a global study. Cancer Sci, 111:3726-3738, 2020
23. Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, Mizugaki H, Murakami H, Fujisaka Y, Kozuki T, Nakamura K, Nagasaka Y, Kawasaki M, Yamada T, Machida R, Kuchiba A, Ohe Y, Yamamoto N. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol, 21:843-850, 2020
24. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol, 21:1346-1358, 2020
25. Yatabe Y, Sunami K, Goto K, Nishio K, Aragane N, Ikeda S, Inoue A, Kinoshita I, Kimura H, Sakamoto T, Satouchi M, Shimizu J, Tsuta K, Toyooka S, Nishino K, Hatanaka Y, Matsumoto S, Mikubo M, Yokose T, Dosaka-Akita H. Multiplex gene-panel testing for lung cancer patients. Pathol Int, 70:921-931, 2020
26. Yamazaki S, Su Y, Maruyama A, Makinoshima H, Suzuki J, Tsuboi M, Goto K, Ochiai A, Ishii G. Uptake of collagen type I via macropinocytosis cause mTOR activation and anti-cancer drug resistance. Biochem Biophys Res Commun, 526:191-198, 2020
27. Kenmotsu H, Yamamoto N, Yamanaka T, Yoshiya K, Takahashi T, Ueno T, Goto K, Daga H, Ikeda N, Sugio K, Seto T, Toyooka S, Date H, Mitsudomi T, Okamoto I, Yokoi K, Saka H, Okamoto H, Takiguchi Y, Tsuboi M. Randomized Phase III Study of Pemetrexed Plus Cisplatin Versus Vinorelbine Plus Cisplatin for Completely Resected Stage II to IIIA Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol, 38:2187-2196, 2020
28. Sato K, Mimaki S, Yamashita R, Togashi Y, Naito T, Udagawa H, Katsumata S, Nakasone S, Miyoshi T, Tane K, Aokage K, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Goto K, Tsuboi M, Tsuchihara K, Ishii G. Association between the mutational smoking signature and the immune microenvironment in lung adenocarcinoma. Lung Cancer, 147:12-20, 2020
29. Nishio M, Seto T, Reck M, Garon EB, Chiu CH, Yoh K, Imamura F, Park K, Shih JY, Visseren-Grul C, Frimodt-Moller B, Zimmermann A, Homma G, Enatsu S, Nakagawa K. Ramucirumab or placebo plus erlotinib in EGFR-mutated, metastatic non-small-cell lung cancer: East Asian subset of RELAY. Cancer Sci, 111:4510-4525, 2020
30. Matsui R, Suzuki K, Takiguchi T, Nishio M, Koike T, Hayashi T, Seto T, Kogure Y, Nogami N, Fujiwara K, Kaneda H, Harada T, Shimizu S, Kimura M, Kenmotsu H, Shimokawa M, Goto K. 5-Hydroxytryptamine-3 receptor antagonist and dexamethasone as prophylaxis for chemotherapy-induced nausea and vomiting during moderately emetic chemotherapy for solid tumors: a multicenter, prospective, observational study. BMC Pharmacol Toxicol, 21:72, 2020
31. Nishiyama A, Takeuchi S, Adachi Y, Otani S, Tanimoto A, Sasaki M, Matsumoto S, Goto K, Yano S. MET amplification results in heterogeneous responses to osimertinib in EGFR-mutant lung cancer treated with erlotinib. Cancer Sci, 111:3813-3823, 2020
32. Yamamoto N, Hayashi H, Planchard D, Moran T, Gregorc V, Dowell J, Sakai H, Yoh K, Nishio M, Cortot AB, Benhadji KA, Soni N, Huang J, Makris L, Cedres S. A randomized, phase 2 study of deoxyuridine triphosphatase inhibitor, TAS-114, in combination with S-1 versus S-1 alone in patients with advanced non-small-cell lung cancer. Invest New Drugs, 38:1588-1597, 2020
33. Yoh K, Atagi S, Reck M, Garon EB, Ponce Aix S, Moro-Sibilot D, Winfree KB, Frimodt-Moller B, Zimmermann A, Visseren-Grul C, Nakagawa K. Patient-reported outcomes in RELAY, a phase 3 trial of ramucirumab plus erlotinib versus placebo plus erlotinib in untreated EGFR-mutated metastatic non-small-cell lung cancer. Curr Med Res Opin, 36:1667-1675, 2020
34. Uchio N, Unuma A, Kakumoto T, Osaki M, Zenke Y, Sakuta K, Kubota A, Uesaka Y, Toda T, Shimizu J. Pembrolizumab on pre-existing inclusion body myositis: a case report. BMC Rheumatol, 4:48, 2020
35. Umemura S, Zhu J, Chahine JJ, Kallakury B, Chen V, Kim IK, Zhang YW, Goto K, He Y, Giaccone G. Downregulation of CYLD promotes IFN-γ mediated PD-L1 expression in thymic epithelial tumors. Lung Cancer, 147:221-228, 2020
36. Kunimasa K, Matsumoto S, Nishino K, Nakamura H, Kuhara H, Tamiya M, Inoue T, Kawamura T, Kawachi H, Kuno K, Kimura T, Maniwa T, Okami J, Nakatsuka SI, Goto K, Kumagai T. Improvement strategies for successful next-generation sequencing analysis of lung cancer. Future Oncol, 16:1597-1606, 2020
37. Nakamura M, Kageyama SI, Udagawa H, Zenke Y, Yoh K, Niho S, Hojo H, Motegi A, Kirita K, Matsumoto S, Goto K, Akimoto T. Differences in failure patterns according to the EGFR mutation status after proton beam therapy for early stage non-small cell lung cancer. Radiother Oncol, 149:14-17, 2020
38. Ikeda T, Takemoto S, Senju H, Gyotoku H, Taniguchi H, Shimada M, Dotsu Y, Umeyama Y, Tomono H, Kitazaki T, Fukuda M, Soda H, Yamaguchi H, Fukuda M, Mukae H. Amrubicin in previously treated patients with malignant pleural mesothelioma: A phase II study. Thorac Cancer, 11:1972-1978, 2020
39. Udagawa H, Kirita K, Naito T, Nomura S, Ishibashi M, Matsuzawa R, Hisakane K, Usui Y, Matsumoto S, Yoh K, Niho S, Ishii G, Goto K. Feasibility and utility of transbronchial cryobiopsy in precision medicine for lung cancer: Prospective single-arm study. Cancer Sci, 111:2488-2498, 2020
40. Wakuda K, Yamaguchi H, Kenmotsu H, Fukuda M, Takeshita M, Suetsugu T, Kirita K, Ebi N, Hataji O, Miura S, Chibana K, Okamoto I, Yoshimura K, Nakagawa K, Yamamoto N, Sugio K. A phase II study of Osimertinib for patients with radiotherapy-na?ve CNS metastasis of non-small cell lung cancer: treatment rationale and protocol design of the OCEAN study (LOGIK 1603/WJOG 9116L). BMC Cancer, 20:370, 2020
41. Nishio M, Kato T, Niho S, Yamamoto N, Takahashi T, Nogami N, Kaneda H, Fujita Y, Wilner K, Yoshida M, Isozaki M, Wada S, Tsuji F, Nakagawa K.Safety and efficacy of first-line dacomitinib in Japanese patients with advanced non-small cell lung cancer. Cancer Sci, 111, 5, 1724-38, 2020.
42. Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, Goto K, Ohe Y, Mann H, Thress KS, Frigault MM, Vishwanathan K, Ghiorghiu D, Ramalingam SS, Ahn MJ. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol, 31:507-516, 2020
43. Yoh K, Takamochi K, Shukuya T, Hishida T, Tsuboi M, Sakurai H, Goto Y, Yoshida K, Ohde Y, Okumura S, Ohashi Y, Kunitoh H. Corrigendum to: Pattern of care in adjuvant therapy for resected Stage I non-small cell lung cancer: real-world data from Japan. Jpn J Clin Oncol, 50:481, 2020
44. Yamamoto N, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Yamanaka T, Tanaka M, Takahashi K, Fukuoka M.Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: Survival follow-up results of the randomized JO25567 study. Lung Cancer, 151, 20-24, 2021.
45. Nishio M, Kato T, Niho S, Yamamoto N, Takahashi T, Nogami N, Kaneda H, Fujita Y, Wilner K, Yoshida M, Isozaki M, Wada S, Tsuji F, Nakagawa K.Safety and efficacy of first-line dacomitinib in Japanese patients with advanced non-small cell lung cancer. Cancer Sci, 111, 5, 1724-38, 2020.
