Annual Report 2020
Department of Gastrointestinal Oncology
Takayuki Yoshino, Toshihiko Doi, Takashi Kojima, Kouhei Shitara, Nozomu Fuse, Hiroya Taniguchi, Yasutoshi Kuboki, Akihito Kawazoe, Daisuke Kotani, Yoshiaki Nakamura, Masataka Yagisawa, Keigo Chida, Saori Mishima
Introduction
In 2019, approximately 770 gastrointestinal (GI) cancer patients were treated by staff oncologists and skilled residents in the Department of GI Oncology, which focuses on the optimal chemotherapy with or without radiation for the treatment of GI cancers.
The Team and What We Do
Interdivisional tumor board conferences with the Surgical/Radiation Oncology Divisions are held regularly to review the current treatment for each patient and to discuss further treatment strategies. Our activities for each type of GI cancer in 2020 are shown in Table 1. There are ongoing clinical trials that consist of 66 Phase I trials, including globally first-in-class (FIC), first-in-human (FIH), investigational new drugs (INDs); 62 Phase II/III clinical trials in order to approve the INDs; and 20 investigator-initiated clinical trials (IITs). In addition, young skilled residents in their early 30s have become principal investigators for several IITs.
Table 1. Number of new patients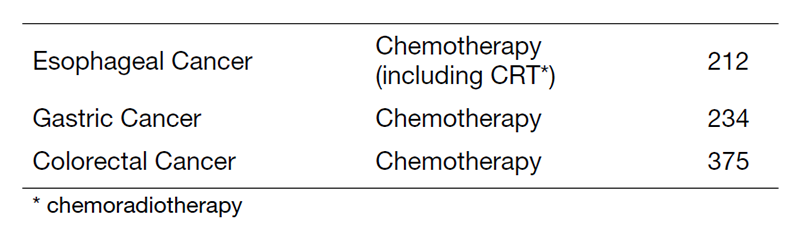

Research activities
Phase I
Our department has focused more on early-stage clinical development of INDs. The number of patients enrolled for First in human studies (FIH) has been increasing recently, especially in studies on immunomodulating agents, antibody-drug conjugate (ADC). Furthermore, new types of cancer therapy, such as cell therapies using chimeric antigen receptor (CAR)-T cells and bispecific T-cell engager (BiTE) antibodies (a complex formed with antibodies against CD3 and antibodies against tumor cell surface antigens), will be more focused on solid tumors, including GI tumors. From the standpoint of genome-based medicine, KRAS-targeting therapy has become more active.
Esophageal Cancer (EC)
The enrollment of the phase I trial of oncolytic virus and pembrolizumab and the phase Ib/II study to investigate the safety, efficacy, and proof-of-concept (POC) of anti-PD-L1 antibody monotherapy following radical chemoradiotherapy are ongoing. An observational study of circulating tumor-DNA in patients with gastrointestinal cancer has also started.
Gastric Cancer (GC)
We reported several researches to clarify the immune profiles in gastric cancer or to investigate biomarkers to predict treatment outcomes after PD-1 blockade as collaborations with the division of cancer immunology. Two global phase III studies (LEAP-015 and INTEGRATE2b) are ongoing based on our investigator-initiated trials of lenvatinib plus pembrolizumab or regorafenib plus nivolumab for gastric cancer. We reported the ratio between PD-1+ CD8 and PD-1+ regulatory T cells in tumor infiltrating lymphocytes as a promising predictive factor for treatment outcomes after PD-blockade. Currently, a prospective phase II trial is ongoing to confirm the findings (TASUKI-88). The enrollment of a phase I trial of photoimmunotherapy plus nivolumab for patients with EGFR expressing gastric cancer are ongoing. Furthermore, phase II trial of neoadjuvant treatment with lenvatinib plus pembrolizumab or a phase II study of neoadjuvant trastuzumab deruxtecan will be started this year. Our department contributed several international global studies such as a phase III trial of nivolumab plus chemotherapy (Checkmate649) or pembrolizumab (KEYNOTE-061, 062) for gastric cancer, and published these results in major journals, such as the LANCET, JAMA Oncology, and Annals of Oncology as the first/lead author.
Colorectal Cancer (CRC)
We have conducted our initiative SCRUM-Japan platform, which is a nationwide cancer genome screening system using tumor-tissue (GI-SCREEN and MONSTAR-SCREEN) and circulating tumor DNA-based NGS screening (GOZILA project) for metastatic solid tumors. Utilizing these screening systems, the umbrella type of IITs for metastatic CRC or solid tumor patients with HER2 amplification, BRAF non-V600E mutation, MET amplification, tumor mutational burden-high, ROS1 fusion, and BRCA mutation are ongoing. We have also started IITs in resectable colorectal cancer (CIRCULATE-Japan project) to stratify them according to recurrence risk based on a Signatera assay for detecting minimal residual disease.
Education
Our residents learn the latest evidence-based medicine and apply this knowledge pragmatically to enhance care for patients with GI cancers, and eventually gain qualifications as comprehensive GI oncologists through the daily practice and the direct training from our staff. Accordingly, our staff actively provide a pile of valuable opportunities to polish the skill in various chemotherapies, especially in collaboration with Department of Experimental Therapeutics as well as diagnostic and therapeutic endoscopies in collaboration with Department of Digestive Endoscopy. We regularly hold tumor board meetings and have numerous face-to-face opportunities to liaison with experts in a variety of specialties. We instruct them how to conduct valuable clinical trials, provide the chance to attend international academic conferences, and advise them on the best way to present academic meetings and work on many high-impact articles for scholarly journals. To date, our department has led many residents to become “true” skilled GI oncologists who play major roles at leading cancer centers across the country.
Future Prospects
We will continue to provide the best treatment possible for cancer patients, the best education for residents, and aim to perform the following activities:
1) To provide more of the latest, cutting-edge medicine to cancer patients and to foster more the next generation of skilled GI oncologists.
2) To achieve medical innovation from Japan, we aim to play leading roles in the clinical development of INDs by contributing to various types of clinical trials including FIC, FIH early trials, IITs with proof-of-concept, and international clinical trials.
3) To enhance our research activity, we will establish research networks with cutting-edge researchers globally.
List of papers published in 2020
Journal
1. Kawazoe A, Shitara K, Boku N, Yoshikawa T, Terashima M. Current status of immunotherapy for advanced gastric cancer. Jpn J Clin Oncol, 51:20-27, 2021
2. Hasegawa H, Taniguchi H, Nakamura Y, Kato T, Fujii S, Ebi H, Shiozawa M, Yuki S, Masuishi T, Kato K, Izawa N, Moriwaki T, Oki E, Kagawa Y, Denda T, Nishina T, Tsuji A, Hara H, Esaki T, Nishida T, Kawakami H, Sakamoto Y, Miki I, Okamoto W, Yamazaki K, Yoshino T. FMS-like tyrosine kinase 3 (FLT3) amplification in patients with metastatic colorectal cancer. Cancer Sci, 112:314-322, 2021
3. Doi T, Fujiwara Y, Shitara K, Shimizu T, Yonemori K, Matsubara N, Ohno I, Kogawa T, Naito Y, Leopold L, Munteanu M, Yatsuzuka N, Han SR, Samkari A, Yamamoto N. The safety and tolerability of epacadostat alone and in combination with pembrolizumab in patients with advanced solid tumors: results from a first-in-Japanese phase I study (KEYNOTE-434). Invest New Drugs, 39:152-162, 2021
4. Powderly J, Spira A, Kondo S, Doi T, Luke JJ, Rasco D, Gao B, Tanner M, Cassier PA, Gazzah A, Italiano A, Tosi D, Afar DE, Parikh A, Engelhardt B, Englert S, Lambert SL, Kasichayanula S, Mensing S, Menon R, Vosganian G, Tolcher A. Model Informed Dosing Regimen and Phase I Results of the Anti-PD-1 Antibody Budigalimab (ABBV-181). Clin Transl Sci, 14:277-287, 2021
5. Sunami K, Naito Y, Aimono E, Amano T, Ennishi D, Kage H, Kanai M, Komine K, Koyama T, Maeda T, Morita S, Sakai D, Kohsaka S, Tsuchihara K, Yoshino T. The initial assessment of expert panel performance in core hospitals for cancer genomic medicine in Japan. Int J Clin Oncol, 26:443-449, 2021
6. Naito Y, Aburatani H, Amano T, Baba E, Furukawa T, Hayashida T, Hiyama E, Ikeda S, Kanai M, Kato M, Kinoshita I, Kiyota N, Kohno T, Kohsaka S, Komine K, Matsumura I, Miura Y, Nakamura Y, Natsume A, Nishio K, Oda K, Oda N, Okita N, Oseto K, Sunami K, Takahashi H, Takeda M, Tashiro S, Toyooka S, Ueno H, Yachida S, Yoshino T, Tsuchihara K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int J Clin Oncol, 26:233-283, 2021
7. Minami H, Doi T, Toyoda M, Imamura Y, Kiyota N, Mitsuma A, Shimokata T, Naito Y, Matsubara N, Tajima T, Tokushige K, Ishihara K, Cameron S, Ando Y. Phase I study of the antiprogrammed cell death-1 Ab spartalizumab (PDR001) in Japanese patients with advanced malignancies. Cancer Sci, 112:725-733, 2021
8. Koganemaru S, Shitara K. Antibody-drug conjugates to treat gastric cancer. Expert Opin Biol Ther, 21:923-930, 2021
9. Hatake K, Chou T, Doi T, Terui Y, Kato H, Hirose T, Seo S, Pourdehnad M, Ogaki Y, Fujimoto H, Hagner PR, Yamamoto K. Phase I, multicenter, dose-escalation study of avadomide in adult Japanese patients with advanced malignancies. Cancer Sci, 112:331-338, 2021
10. Kotani D, Shitara K. Trastuzumab deruxtecan for the treatment of patients with HER2-positive gastric cancer. Ther Adv Med Oncol, 13:1758835920986518, 2021
11. Kawazoe A, Takahari D, Keisho C, Nakamura Y, Ikeno T, Wakabayashi M, Nomura S, Tamura H, Fukutani M, Hirano N, Saito Y, Kambe M, Sato A, Shitara K. A multicenter phase II study of TAS-114 in combination with S-1 in patients with pretreated advanced gastric cancer (EPOC1604). Gastric Cancer, 24:190-196, 2021
12. Kawazoe A, Ando T, Hosaka H, Fujita J, Koeda K, Nishikawa K, Amagai K, Fujitani K, Ogata K, Watanabe K, Yamamoto Y, Shitara K. Safety and activity of trifluridine/tipiracil and ramucirumab in previously treated advanced gastric cancer: an open-label, single-arm, phase 2 trial. Lancet Gastroenterol Hepatol, 6:209-217, 2021
13. Yamazaki K, Yamanaka T, Shiozawa M, Manaka D, Kotaka M, Gamoh M, Shiomi A, Makiyama A, Munemoto Y, Rikiyama T, Fukunaga M, Ueki T, Shitara K, Shinkai H, Tanida N, Oki E, Sunami E, Ohtsu A, Maehara Y, Yoshino T. Oxaliplatin-based adjuvant chemotherapy duration (3 versus 6 months) for high-risk stage II colon cancer: the randomized phase III ACHIEVE-2 trial. Ann Oncol, 32:77-84, 2021
14. Kashima Y, Togashi Y, Fukuoka S, Kamada T, Irie T, Suzuki A, Nakamura Y, Shitara K, Minamide T, Yoshida T, Taoka N, Kawase T, Wada T, Inaki K, Chihara M, Ebisuno Y, Tsukamoto S, Fujii R, Ohashi A, Suzuki Y, Tsuchihara K, Nishikawa H, Doi T. Potentiality of multiple modalities for single-cell analyses to evaluate the tumor microenvironment in clinical specimens. Sci Rep, 11:341, 2021
15. Akagi K, Oki E, Taniguchi H, Nakatani K, Aoki D, Kuwata T, Yoshino T. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci, 112:1105-1113, 2021
16. Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, Desai J, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Elez E, Gollerkeri A, Maharry K, Christy-Bittel J, Kopetz S. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol, 39:273-284, 2021
17. Iveson TJ, Sobrero AF, Yoshino T, Souglakos I, Ou FS, Meyers JP, Shi Q, Grothey A, Saunders MP, Labianca R, Yamanaka T, Boukovinas I, Hollander NH, Galli F, Yamazaki K, Georgoulias V, Kerr R, Oki E, Lonardi S, Harkin A, Rosati G, Paul J. Duration of Adjuvant Doublet Chemotherapy (3 or 6 months) in Patients With High-Risk Stage II Colorectal Cancer. J Clin Oncol, 39:1691, 2021
18. Cohen R, Taieb J, Fiskum J, Yothers G, Goldberg R, Yoshino T, Alberts S, Allegra C, de Gramont A, Seitz JF, O'Connell M, Haller D, Wolmark N, Erlichman C, Zaniboni A, Lonardi S, Kerr R, Grothey A, Sinicrope FA, André T, Shi Q. Microsatellite Instability in Patients With Stage III Colon Cancer Receiving Fluoropyrimidine With or Without Oxaliplatin: An ACCENT Pooled Analysis of 12 Adjuvant Trials. J Clin Oncol, 39:642-651, 2021
19. Lenz HJ, Argiles G, Yoshino T, Tejpar S, Ciardiello F, Braunger J, Salnikov AV, Gabrielyan O, Schmid R, Höfler J, Kitzing T, Van Cutsem E. Association of Consensus Molecular Subtypes and Molecular Markers With Clinical Outcomes in Patients With Metastatic Colorectal Cancer: Biomarker Analyses From LUME-Colon 1. Clin Colorectal Cancer, 20:84-95.e8, 2021
20. Takahashi T, Yamazaki K, Oki E, Shiozawa M, Mitsugi K, Makiyama A, Nakamura M, Ojima H, Kagawa Y, Matsuhashi N, Okuda H, Asayama M, Yuasa Y, Shimada Y, Manaka D, Watanabe J, Oba K, Yoshino T, Yoshida K, Maehara Y. Phase II study of trifluridine/tipiracil plus bevacizumab by RAS mutation status in patients with metastatic colorectal cancer refractory to standard therapies: JFMC51-1702-C7. ESMO Open, 6:100093, 2021
21. Fujitani K, Shitara K, Takashima A, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Kimura Y, Amagai K, Hosaka H, Komatsu Y, Shimada K, Kawabata R, Ohdan H, Kodera Y, Nakamura M, Nakajima TE, Miyata Y, Moriwaki T, Kusumoto T, Nishikawa K, Ogata K, Shimura M, Morita S, Koizumi W. Effect of early tumor response on the health-related quality of life among patients on second-line chemotherapy for advanced gastric cancer in the ABSOLUTE trial. Gastric Cancer, 24:467-476, 2021
22. Fujitani K, Shitara K, Takashima A, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Kimura Y, Amagai K, Hosaka H, Komatsu Y, Shimada K, Kawabata R, Ohdan H, Kodera Y, Nakamura M, Nakajima TE, Miyata Y, Moriwaki T, Kusumoto T, Nishikawa K, Ogata K, Shimura M, Morita S, Koizumi W. Correction to: Effect of early tumor response on the health-related quality of life among patients on second-line chemotherapy for advanced gastric cancer in the ABSOLUTE trial. Gastric Cancer, 24:477-478, 2021
23. Kadota T, Ikematsu H, Sasaki T, Saito Y, Ito M, Mizutani T, Ogawa G, Shitara K, Ito Y, Kushima R, Kanemitsu Y, Muto M. Protocol for a single-arm confirmatory trial of adjuvant chemoradiation for patients with high-risk rectal submucosal invasive cancer after local resection: Japan Clinical Oncology Group Study JCOG1612 (RESCUE study). BMJ Open, 10:e034947, 2020
24. Kato K, Doki Y, Ura T, Hamamoto Y, Kojima T, Tsushima T, Hironaka S, Hara H, Kudo T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Kitagawa Y. Long-term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci, 111:1676-1684, 2020
25. Nakajima TE, Yamaguchi K, Boku N, Hyodo I, Mizusawa J, Hara H, Nishina T, Sakamoto T, Shitara K, Shinozaki K, Katayama H, Nakamura S, Muro K, Terashima M. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer, 23:677-688, 2020
26. Kobayashi S, Takahashi S, Takahashi N, Masuishi T, Shoji H, Shinozaki E, Yamaguchi T, Kojima M, Gotohda N, Nomura S, Yoshino T, Taniguchi H. Survival Outcomes of Resected BRAF V600E Mutant Colorectal Liver Metastases: A Multicenter Retrospective Cohort Study in Japan. Ann Surg Oncol, 27:3307-3315, 2020
27. Doi T, Boku N, Onozawa Y, Takahashi K, Kawaguchi O, Ohtsu A. Phase I dose-escalation study of the safety, tolerability, and pharmacokinetics of aflibercept in combination with S-1 in Japanese patients with advanced solid malignancies. Invest New Drugs, 38:1390-1399, 2020
28. Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, Esaki T, Komatsu Y, Kawamoto Y, Takahashi N, Ueno M, Kagawa Y, Nishina T, Kato T, Yamamoto Y, Furuse J, Denda T, Kawakami H, Oki E, Nakajima T, Nishida N, Yamaguchi K, Yasui H, Goto M, Matsuhashi N, Ohtsubo K, Yamazaki K, Tsuji A, Okamoto W, Tsuchihara K, Yamanaka T, Miki I, Sakamoto Y, Ichiki H, Hata M, Yamashita R, Ohtsu A, Odegaard JI, Yoshino T. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med, 26:1859-1864, 2020
29. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol, 38:4138-4148, 2020
30. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med, 382:2419-2430, 2020
31. Doi T, Matsubara N, Kawai A, Naka N, Takahashi S, Uemura H, Yamamoto N. Phase I study of TAS-115, a novel oral multi-kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs, 38:1175-1185, 2020
32. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol, 21:1346-1358, 2020
33. Kang YK, Bang YJ, Kondo S, Chung HC, Muro K, Dussault I, Helwig C, Osada M, Doi T. Safety and Tolerability of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Asian Patients with Pretreated Recurrent or Refractory Gastric Cancer. Clin Cancer Res, 26:3202-3210, 2020
34. Kondo S, Tajimi M, Funai T, Inoue K, Asou H, Ranka VK, Wacheck V, Doi T. Phase 1 dose-escalation study of a novel oral PI3K/mTOR dual inhibitor, LY3023414, in patients with cancer. Invest New Drugs, 38:1836-1845, 2020
35. Kuwata T, Wakabayashi M, Hatanaka Y, Morii E, Oda Y, Taguchi K, Noguchi M, Ishikawa Y, Nakajima T, Sekine S, Nomura S, Okamoto W, Fujii S, Yoshino T. Impact of DNA integrity on the success rate of tissue-based next-generation sequencing: Lessons from nationwide cancer genome screening project SCRUM-Japan GI-SCREEN. Pathol Int, 70:932-942, 2020
36. Shitara K, Yamazaki K, Tsushima T, Naito T, Matsubara N, Watanabe M, Sarholz B, Johne A, Doi T. Phase I trial of the MET inhibitor tepotinib in Japanese patients with solid tumors. Jpn J Clin Oncol, 50:859-866, 2020
37. Yoshino T, Pentheroudakis G, Mishima S, Overman MJ, Yeh KH, Baba E, Naito Y, Calvo F, Saxena A, Chen LT, Takeda M, Cervantes A, Taniguchi H, Yoshida K, Kodera Y, Kitagawa Y, Tabernero J, Burris H, Douillard JY. JSCO-ESMO-ASCO-JSMO-TOS: international expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol, 31:861-872, 2020
38. Sasaki A, Harano K, Kogawa T, Matsubara N, Naito Y, Hosono A, Mukai H, Yoshino T, Mukohara T. Intestinal Perforation due to Neutropenic Enterocolitis in a Patient Treated with Bevacizumab for Ovarian Cancer. Case Rep Oncol Med, 2020:7231358, 2020
39. Kawazoe A, Kuboki Y, Bando H, Fukuoka S, Kojima T, Naito Y, Iino S, Yodo Y, Doi T, Shitara K, Yoshino T. Phase 1 study of napabucasin, a cancer stemness inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol, 85:855-862, 2020
40. Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H. An Oncogenic Alteration Creates a Microenvironment that Promotes Tumor Progression by Conferring a Metabolic Advantage to Regulatory T Cells. Immunity, 53:187-203.e8, 2020
41. Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, Hara H, Baba H, Tsuda M, Hosaka H, Kawakami H, Oshima T, Omuro Y, Machida N, Esaki T, Yoshida K, Nishina T, Komatsu Y, Han SR, Shiratori S, Shitara K. Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: Cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer, 129:97-106, 2020
42. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol, 38:2053-2061, 2020
43. Doi T, Fujiwara Y, Koyama T, Ikeda M, Helwig C, Watanabe M, Vugmeyster Y, Kudo M. Phase I Study of the Bifunctional Fusion Protein Bintrafusp Alfa in Asian Patients with Advanced Solid Tumors, Including a Hepatocellular Carcinoma Safety-Assessment Cohort. Oncologist, 25:e1292-e1302, 2020
44. Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N, Higuchi T, Sato A, Kuwata T, Shitara K. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol, 21:1057-1065, 2020
45. Kubota Y, Kawazoe A, Sasaki A, Mishima S, Sawada K, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Ishii G, Kuwata T, Shitara K. The Impact of Molecular Subtype on Efficacy of Chemotherapy and Checkpoint Inhibition in Advanced Gastric Cancer. Clin Cancer Res, 26:3784-3790, 2020
46. Ishii T, Kawazoe A, Sasaki A, Mishima S, Kentaro S, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Kuwata T, Ishii G, Shitara K. Clinical and molecular factors for selection of nivolumab or irinotecan as third-line treatment for advanced gastric cancer. Ther Adv Med Oncol, 12:1758835920942377, 2020
47. Sasaki A, Kawazoe A, Eto T, Okunaka M, Mishima S, Sawada K, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Akimoto T, Shitara K. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer. ESMO Open, 4:2020
48. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, Yuki S, Wakabayashi M, Nomura S, Sato A, Kuwata T, Kawazu M, Mano H, Togashi Y, Nishikawa H, Yoshino T. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin Cancer Res, 26:5887-5894, 2020
49. Yoshino T, Lenz HJ. Reply to the letter to the editor 'Neutropenia in metastatic colorectal cancer receiving trifluridine/tipiracil' by Colloca et al. Ann Oncol, 31:1085-1087, 2020
50. Sasaki A, Nakamura Y, Togashi Y, Kuno H, Hojo H, Kageyama S, Nakamura N, Takashima K, Kadota T, Yoda Y, Mishima S, Sawada K, Kotani D, Kawazoe A, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Yano T, Kobayashi T, Akimoto T, Nishikawa H, Shitara K. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer, 23:893-903, 2020
51. Mishima S, Kawazoe A, Shitara K. Safety of pembrolizumab in recurrent or advanced gastric cancer expressing PD-L1 refractory to platinum and fluoropyrimidine. Expert Opin Drug Saf, 19:1063-1068, 2020
52. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol, 6:1571-1580, 2020
53. Nakamura Y, Sasaki A, Yukami H, Jogo T, Kawazoe A, Kuboki Y, Taniguchi H, Yamashita R, Kuwata T, Ozawa M, Nakamura M, Yoshino T, Shitara K. Emergence of Concurrent Multiple EGFR Mutations and MET Amplification in a Patient With EGFR-Amplified Advanced Gastric Cancer Treated With Cetuximab. JCO Precis Oncol, 4:2020
54. Okunaka M, Kotani D, Demachi K, Kawazoe A, Yoshino T, Kawasaki T, Shitara K. Retrospective cohort study of nanoparticle albumin-bound paclitaxel plus ramucirumab versus paclitaxel plus ramucirumab as second-line treatment in patients with advanced gastric cancer. BMC Cancer, 20:1111, 2020
55. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, Rugo HS, de Braud F, Varga AI, Hansen A, Wang H, Krishnan S, Norwood KG, Doi T. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer, 147:2190-2198, 2020
56. Kobayashi S, Takahashi S, Yoshino T, Taniguchi H. ASO Author Reflections: The Moment That BRAF V600E Mutation Starts Evolving into "Precision Oncosurgery" in Colorectal Liver Metastases. Ann Surg Oncol, 27:3316-3317, 2020
57. Kondo S, Tajimi M, Funai T, Inoue K, Asou H, Ranka VK, Wacheck V, Doi T. Correction to: Phase 1 dose-escalation study of a novel oral PI3K/mTOR dual inhibitor, LY3023414, in patients with cancer. Invest New Drugs, 38:1846, 2020
58. Park YH, Senkus-Konefka E, Im SA, Pentheroudakis G, Saji S, Gupta S, Iwata H, Mastura MY, Dent R, Lu YS, Yin Y, Smruti BK, Toyama T, Malwinder S, Lee SC, Tseng LM, Kim JH, Kim TY, Suh KJ, Cardoso F, Yoshino T, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with early breast cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol, 31:451-469, 2020
59. Salem ME, Yin J, Goldberg RM, Pederson LD, Wolmark N, Alberts SR, Taieb J, Marshall JL, Lonardi S, Yoshino T, Kerr RS, Yothers G, Grothey A, Andre T, De Gramont A, Shi Q. Evaluation of the change of outcomes over a 10-year period in patients with stage III colon cancer: pooled analysis of 6501 patients treated with fluorouracil, leucovorin, and oxaliplatin in the ACCENT database. Ann Oncol, 31:480-486, 2020
60. Gelderblom H, Jones RL, George S, Valverde Morales C, Benson C, Jean-Yves Blay, Renouf DJ, Doi T, Le Cesne A, Leahy M, Hertle S, Aimone P, Brandt U, Sch?ffski P. Imatinib in combination with phosphoinositol kinase inhibitor buparlisib in patients with gastrointestinal stromal tumour who failed prior therapy with imatinib and sunitinib: a Phase 1b, multicentre study. Br J Cancer, 122:1158-1165, 2020
61. Cohen R, Vernerey D, Bellera C, Meurisse A, Henriques J, Paoletti X, Rousseau B, Alberts S, Aparicio T, Boukovinas I, Gill S, Goldberg RM, Grothey A, Hamaguchi T, Iveson T, Kerr R, Labianca R, Lonardi S, Meyerhardt J, Paul J, Punt CJA, Saltz L, Saunders MP, Schmoll HJ, Shah M, Sobrero A, Souglakos I, Taieb J, Takashima A, Wagner AD, Ychou M, Bonnetain F, Gourgou S, Yoshino T, Yothers G, de Gramont A, Shi Q, Andr? T. Guidelines for time-to-event end-point definitions in adjuvant randomised trials for patients with localised colon cancer: Results of the DATECAN initiative. Eur J Cancer, 130:63-71, 2020
62. Tsurutani J, Iwata H, Krop I, J?nne PA, Doi T, Takahashi S, Park H, Redfern C, Tamura K, Wise-Draper TM, Saito K, Sugihara M, Singh J, Jikoh T, Gallant G, Li BT. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov, 10:688-701, 2020
63. Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, Bergsland E, Shah M, Fakih M, Takahashi S, Piha-Paul SA, O'Neil B, Thomas S, Lolkema MP, Chen M, Ibrahim N, Norwood K, Hadoux J. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin Cancer Res, 26:2124-2130, 2020
64. Middleton G, Yang Y, Campbell CD, Andr? T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, Hollebecque A, McRee AJ, Siena S, Gordon MS, Tabernero J, Yaeger R, O'Dwyer PJ, De Vos F, Van Cutsem E, Millholland JM, Brase JC, Rangwala F, Gasal E, Corcoran RB. BRAF-Mutant Transcriptional Subtypes Predict Outcome of Combined BRAF, MEK, and EGFR Blockade with Dabrafenib, Trametinib, and Panitumumab in Patients with Colorectal Cancer. Clin Cancer Res, 26:2466-2476, 2020
65. Grothey A, Blay JY, Pavlakis N, Yoshino T, Bruix J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat Rev, 86:101993, 2020
66. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol, 38:1887-1896, 2020
67. Miyo M, Kato T, Yoshino T, Yamanaka T, Bando H, Satake H, Yamazaki K, Taniguchi H, Oki E, Kotaka M, Oba K, Miyata Y, Muro K, Komatsu Y, Baba H, Tsuji A. Protocol of the QUATTRO-II study: a multicenter randomized phase II study comparing CAPOXIRI plus bevacizumab with FOLFOXIRI plus bevacizumab as a first-line treatment in patients with metastatic colorectal cancer. BMC Cancer, 20:687, 2020
68. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Reply to T.J.A. Dekker. J Clin Oncol, 38:3351-3352, 2020
69. Nishida T, Doi T. A new approach to refractory gastrointestinal stromal tumours with diverse acquired mutations. Lancet Oncol, 21:864-865, 2020
70. Tabernero J, Alsina M, Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, Ghidini M, Faustino C, Gorbunova V, Zhavrid E, Nishikawa K, Ando T, Yal??n ?, Van Cutsem E, Sabater J, Skanji D, Leger C, Amellal N, Ilson DH. Health-related quality of life associated with trifluridine/tipiracil in heavily pretreated metastatic gastric cancer: results from TAGS. Gastric Cancer, 23:689-698, 2020
71. Tsumura R, Koga Y, Hamada A, Kuwata T, Sasaki H, Doi T, Aikawa K, Ohashi A, Katano I, Ikarashi Y, Ito M, Ochiai A. Report of the use of patient-derived xenograft models in the development of anticancer drugs in Japan. Cancer Sci, 111:3386-3394, 2020
72. Sato D, Motegi A, Kadota T, Kojima T, Bando H, Shinmura K, Hori K, Yoda Y, Oono Y, Zenda S, Ikematsu H, Akimoto T, Yano T. Therapeutic results of proton beam therapy with concurrent chemotherapy for cT1 esophageal cancer and salvage endoscopic therapy for local recurrence. Esophagus, 17:305-311, 2020
73. Mehnert JM, Bergsland E, O'Neil BH, Santoro A, Schellens JHM, Cohen RB, Doi T, Ott PA, Pishvaian MJ, Puzanov I, Aung KL, Hsu C, Le Tourneau C, Hollebecque A, ?lez E, Tamura K, Gould M, Yang P, Stein K, Piha-Paul SA. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer, 126:3021-3030, 2020
74. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med, 383:1207-1217, 2020
75. Izawa N, Shitara K, Yonesaka K, Yamanaka T, Yoshino T, Sunakawa Y, Masuishi T, Denda T, Yamazaki K, Moriwaki T, Okuda H, Kondoh C, Nishina T, Makiyama A, Baba H, Yamaguchi H, Nakamura M, Hyodo I, Muro K, Nakajima TE. Early Tumor Shrinkage and Depth of Response in the Second-Line Treatment for KRAS exon2 Wild-Type Metastatic Colorectal Cancer: An Exploratory Analysis of the Randomized Phase 2 Trial Comparing Panitumumab and Bevacizumab in Combination with FOLFIRI (WJOG6210G). Target Oncol, 15:623-633, 2020
76. Sobrero AF, Puccini A, Shi Q, Grothey A, Andr? T, Shields AF, Souglakos I, Yoshino T, Iveson T, Ceppi M, Bruzzi P. A new prognostic and predictive tool for shared decision making in stage III colon cancer. Eur J Cancer, 138:182-188, 2020
77. Argil?s G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 31:1291-1305, 2020
78. Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, Kinoshita T. Erratum: Author's Affiliation Correction. Type II human epidermal growth factor receptor heterogeneity is a poor prognosticator for type II human epidermal growth factor receptor positive gastric cancer (World J Clin Cases 2019; Aug 6; 7 (15): 1964-1977). World J Clin Cases, 8:5494-5495, 2020
79. Satake H, Kato T, Oba K, Kotaka M, Kagawa Y, Yasui H, Nakamura M, Watanabe T, Matsumoto T, Kii T, Terazawa T, Makiyama A, Takano N, Yokota M, Okita Y, Matoba K, Hasegawa H, Tsuji A, Komatsu Y, Yoshino T, Yamazaki K, Mishima H, Oki E, Nagata N, Sakamoto J. Phase Ib/II Study of Biweekly TAS-102 in Combination with Bevacizumab for Patients with Metastatic Colorectal Cancer Refractory to Standard Therapies (BiTS Study). Oncologist, 25:e1855-e1863, 2020
80. Andr? T, Meyerhardt J, Iveson T, Sobrero A, Yoshino T, Souglakos I, Grothey A, Niedzwiecki D, Saunders M, Labianca R, Yamanaka T, Boukovinas I, Vernerey D, Meyers J, Harkin A, Torri V, Oki E, Georgoulias V, Taieb J, Shields A, Shi Q. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol, 21:1620-1629, 2020
81. Andr? T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med, 383:2207-2218, 2020
82. Sunakawa Y, Nakamura M, Ishizaki M, Kataoka M, Satake H, Kitazono M, Yanagisawa H, Kawamoto Y, Kuramochi H, Ohori H, Nakamura M, Maeda F, Komeno C, Sonezaki T, Takeuchi M, Fujii M, Yoshino T, Tsuji A, Ichikawa W. RAS Mutations in Circulating Tumor DNA and Clinical Outcomes of Rechallenge Treatment With Anti-EGFR Antibodies in Patients With Metastatic Colorectal Cancer. JCO Precision Oncology, 4:898-911, 2020
83. Bando H, Kotani D, Tsushima T, Hara H, Kadowaki S, Kato K, Chin K, Yamaguchi K, Kageyama SI, Hojo H, Nakamura M, Tachibana H, Wakabayashi M, Fukutani M, Togashi Y, Fuse N, Nishikawa H, Kojima T . TENERGY: multicenter phase II study of Atezolizumab monotherapy following definitive Chemoradiotherapy with 5-FU plus Cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Cancer, 20:336, 2020
84. Taniguchi H, Yamanaka T, Sakai D, Muro K, Yamazaki K, Nakata S, Kimura H, Ruff P, Kim TW, Peeters M, Price T . Efficacy of Panitumumab and Cetuximab in Patients with Colorectal Cancer Previously Treated with Bevacizumab; a Combined Analysis of Individual Patient Data from ASPECCT and WJOG6510G. . Cancers (Basel), 12:2020
