Annual Report 2020
Department of Hepatobiliary and Pancreatic Surgery
Naoto Gotohda, Shinichiro Takahashi, Shin Kobayashi, Motokazu Sugimoto, Masashi Kudo, Masaru Konishi, Ryo Morisue, Toshiyuki Suzuki, Masatake Taniguchi, Naoki Yagi, Rei Okada, Ryuji Komine, Yusuke Abe, Tatsuki Ishikawa, Kimimasa Sasaki, Yu Shibahara, Kento Mishima, Yu Igata, Manabu Kujiraoka
Introduction
The Department of Hepatobiliary and Pancreatic Surgery consists of six staff surgeons, five chief residents and eight residents. Our department is responsible for the surgical treatment of patients with hepatic, biliary, and pancreatic cancer and duodenal cancer or low-grade malignant tumors. We conduct multidisciplinary treatment in cooperation with the Department of Hepatobiliary and Pancreatic Oncology, the Department of Diagnostic Radiology, and the Department of Radiation Oncology. We conduct less invasive surgery: laparoscopic surgery for patients with liver and pancreatic tumors.
The Team and What We Do
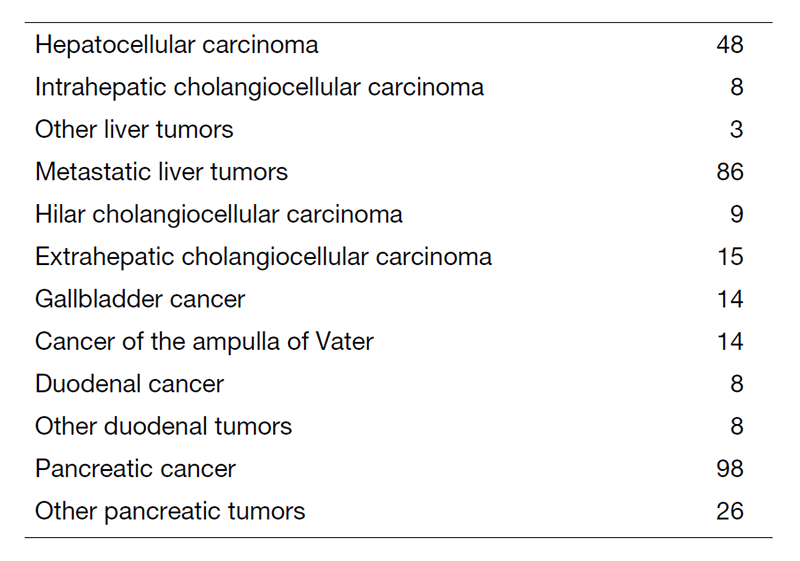
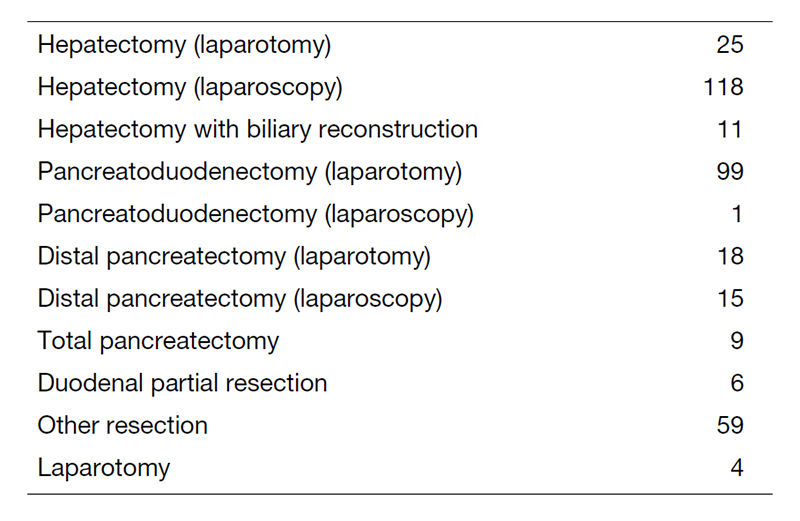
We work with outpatients five days a week and have approximately 20 inpatients. Staff meetings in which we discuss the treatment strategy or the points of surgery for patients are held with staff in the Department of Hepatobiliary and Pancreatic Oncology every Tuesday and Friday. The Cancer Board is held in cooperation with radiologists and medical oncologists every Tuesday. The pathology conference is held monthly with pathologists. In 2020, 365 patients with hepatobiliary and pancreatic diseases underwent surgical treatment. The main diseases are shown in Table 1. Compared with the number of patients in 2019, the number of patients undergoing surgery in our department has increased (Table 2). We performed many laparoscopic surgeries for liver and pancreatic tumors in 2020. Regarding laparoscopic liver surgery, we have already established a position as a leading hospital. Furthermore, we started laparoscopic pancreatoduodenectomy.
Research activities
1. Conversion surgery for pancreatic cancer
Currently, the treatment outcomes of pancreatic cancer patients are improving remarkably with the chemotherapy regimen Gem+nab-PTX or FOLFIRINOX. We attempt conversion surgery for selected patients with borderline resectable or unresectable pancreatic cancer who received chemotherapy. We are currently evaluating good indications for conversion surgery.
2. Function-preserving surgery
Pancreas sparing duodenectomy (PSD) represents an alternative procedure to pancreaticoduodenectomy (PD) for patients with duodenal neoplasms. PSD is a less invasive procedure and has the advantage over PD of preservation of the pancreas. We are attempting to establish a safe procedure for PSD.
3. Evaluation of liver function
Postoperative liver failure is one of the fatal complications after major hepatectomy. We usually evaluate liver function using the indocyanine green retention rate at 15 min (ICG15) test. We are developing an alternative evaluation of liver function employing liver-specific magnetic resonance imaging (MRI) with a gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) contrast agent instead of using the ICG15 test.
4. Cancer genome screening
We conducted clinical studies for cancer genome screening. For patients with resectable HCC, analysis by blood screening (COSMOS-HCC-01) was started in 2020.
“A multicenter proof of concept study for personalized perioperative therapy based on genetic alteration status for resectable oligometastases from colorectal cancer” (PRECISION study) (UMIN000042490) :This is a multicenter prospective observational study to investigate the clinical utility of pre-treatment ctDNA analysis in patients undergoing surgery for colorectal oligometastases with a grant from AMED (20ck0106629h0001). The main eligibility criterion is patients with previously untreated resectable colorectal oligometastases, and the primary endpoint is the detection rate of pre-treatment ctDNA and the positive rate of BRAF V600E in tissue. The period of accrual and follow-up is two years each, and the study started in March 2021.
Clinical trials
1. A phase III trial of S-1 versus observation in patients with resected biliary tract cancer (JCOG1202). Recruitment was completed in 2018.
2. A randomized phase II trial of chemoradiotherapy with S-1 versus gemcitabine and S-1 combination therapy as neoadjuvant treatment in patients with resectable pancreatic cancer (JASPAC04). Recruitment started in 2014.
3. A non-randomized controlled study comparing proton beam therapy and hepatectomy for resectable hepatocellular carcinoma (JCOG1315C). Recruitment started in 2016.
4. Japanese trial - A global study to evaluate the potential benefit of adjuvant chemotherapy for small bowel adenocarcinoma (JCOG1502C). Recruitment started in 2017.
5. A randomized phase II/III study of gemcitabine and nab-paclitaxel therapy versus S-1 and concurrent radiotherapy as neoadjuvant treatment for borderline resectable pancreatic cancer (GABARNANCE Trial). Recruitment started in 2017.
Education
“Board-certified expert surgeons” have a high level of skill in the field of hepatobiliary-pancreatic surgery. To be qualified as a board-certified surgeon, surgeons are required to perform a prescribed number of operations under the guidance of a board-certified instructor. The residents of our department are being trained to receive the certifications by the end of the chief resident course.
Future Prospects
Our goal is the establishment of multidisciplinary treatment for patients with refractory hepatobiliary and pancreatic cancer and the establishment of less invasive surgery for patients with pancreatic cancer and liver cancer.
List of papers published in 2020
Journal
1. Mpekris F, Panagi M, Voutouri C, Martin JD, Samuel R, Takahashi S, Gotohda N, Suzuki T, Papageorgis P, Demetriou P, Pierides C, Koumas L, Costeas P, Kojima M, Ishii G, Constantinidou A, Kataoka K, Cabral H, Stylianopoulos T. Normalizing the Microenvironment Overcomes Vessel Compression and Resistance to Nano-immunotherapy in Breast Cancer Lung Metastasis. Adv Sci (Weinh), 8:2001917, 2021
2. Kobayashi S, Takahashi S, Takahashi N, Masuishi T, Shoji H, Shinozaki E, Yamaguchi T, Kojima M, Gotohda N, Nomura S, Yoshino T, Taniguchi H. Survival Outcomes of Resected BRAF V600E Mutant Colorectal Liver Metastases: A Multicenter Retrospective Cohort Study in Japan. Ann Surg Oncol, 27:3307-3315, 2020
3. Kobayashi S, Takahashi S, Yoshino T, Taniguchi H. ASO Author Reflections: The Moment That BRAF V600E Mutation Starts Evolving into "Precision Oncosurgery" in Colorectal Liver Metastases. Ann Surg Oncol, 27:3316-3317, 2020
4. Gotohda N, Nomura S, Doi M, Karasawa K, Ohki T, Shimizu Y, Inaba Y, Takeda A, Takaki H, Anai H, Ikeda M, Sugimoto M, Akimoto T. Clinical impact of radiofrequency ablation and stereotactic body radiation therapy for colorectal liver metastasis as local therapies for elderly, vulnerable patients. JGH Open, 4:722-728, 2020
5. Umemoto K, Togashi Y, Arai Y, Nakamura H, Takahashi S, Tanegashima T, Kato M, Nishikawa T, Sugiyama D, Kojima M, Gotohda N, Kuwata T, Ikeda M, Shibata T, Nishikawa H. The potential application of PD-1 blockade therapy for early-stage biliary tract cancer. Int Immunol, 32:273-281, 2020
6. Miura M, Fujinami N, Shimizu Y, Mizuno S, Saito K, Suzuki T, Konishi M, Takahashi S, Gotohda N, Suto K, Yoshida T, Nakatsura T. Usefulness of plasma full-length glypican-3 as a predictive marker of hepatocellular carcinoma recurrence after radial surgery. Oncol Lett, 19:2657-2666, 2020
7. Sugimoto M, Kobayashi T, Kobayashi S, Takahashi S, Konishi M, Mitsunaga S, Ikeda M, Gotohda N. Extrapancreatic Nerve Plexus Invasion on Imaging Predicts Poor Survival After Upfront Surgery for Anatomically Resectable Pancreatic Cancer. Pancreas, 49:675-682, 2020
8. Morise Z, Aldrighetti L, Belli G, Ratti F, Belli A, Cherqui D, Tanabe M, Wakabayashi G. Laparoscopic repeat liver resection for hepatocellular carcinoma: a multicentre propensity score-based study. Br J Surg, 107:889-895, 2020
9. Kobayashi S, Beppu T, Honda G, Yamamoto M, Takahashi K, Endo I, Hasegawa K, Kotake K, Itabashi M, Hashiguchi Y, Kotera Y, Sakamoto K, Yamaguchi T, Morita S, Tabuchi K, Miyazaki M, Sugihara K. Survival Benefit of and Indications for Adjuvant Chemotherapy for Resected Colorectal Liver Metastases-a Japanese Nationwide Survey. J Gastrointest Surg, 24:1244-1260, 2020
10. Kojima M, Yamauchi C, Oyamada S, Hojo T, Iwase S, Naito A, Yamano K, Takahashi S, Ochiai A. Assessment of Upper Limb Physiological Features in Patients with Lymphedema After Breast Surgery Using Multiple Instruments. Lymphat Res Biol, 18:239-246, 2020
11. Takahashi D, Kojima M, Morisue R, Sugimoto M, Kobayashi S, Takahashi S, Konishi M, Gotohda N, Ikeda M, Ochiai A. Comparison of morphological features in lymph node metastasis between pancreatic neuroendocrine neoplasms and pancreatic ductal adenocarcinomas. Pancreatology, 20:936-943, 2020
12. Kudo M, Kobayashi T, Gotohda N, Konishi M, Takahashi S, Kobayashi S, Sugimoto M, Okubo S, Martin J, Cabral H, Ishii G, Kojima M. Clinical Utility of Histological and Radiological Evaluations of Tumor Necrosis for Predicting Prognosis in Pancreatic Cancer. Pancreas, 49:634-641, 2020
13. Taniguchi M, Mizuno S, Yoshikawa T, Fujinami N, Sugimoto M, Kobayashi S, Takahashi S, Konishi M, Gotohda N, Nakatsura T. Peptide vaccine as an adjuvant therapy for glypican-3-positive hepatocellular carcinoma induces peptide-specific CTLs and improves long prognosis. Cancer Sci, 111:2747-2759, 2020
14. Nishida Y, Nagatsuma AK, Kojima M, Gotohda N, Ochiai A. Novel stromal biomarker screening in pancreatic cancer patients using the in vitro cancer-stromal interaction model. BMC Gastroenterol, 20:411, 2020
15. Yasuta S, Kobayashi T, Aizawa H, Takahashi S, Ikeda M, Konishi M, Kojima M, Kuno H, Uesaka K, Morinaga S, Miyamoto A, Toyama H, Takakura N, Sugimachi K, Takayama W. Relationship between surgical R0 resectability and findings of peripancreatic vascular invasion on CT imaging after neoadjuvant S-1 and concurrent radiotherapy in patients with borderline resectable pancreatic cancer. BMC Cancer, 20:1184, 2020
