Annual Report 2020
Department of General Internal Medicine, Cardiovascular Medicine
Toshihiko Doi, Keiji Okinaka, Yoichi Naito, Yasutoshi Kuboki, Yusuke Hashimoto, Tomofumi Miura, Nobuhiko Yamauchi, Kensuke Shinmura, Tetsuya Sakai
Introduction
1. General internal medicine
We provide general management across cancer types, support for medical treatment in each department (management for complications, adverse events, etc.), and management aimed at training oncology specialists. As an educational hospital of the Japanese Society of Internal Medicine, we contribute to the training for internal medicine with the aim of nurturing general medical specialists. Based on our experience with immune-related adverse events (irAEs) associated with an increasing number of indications and use of immune checkpoint inhibitors in a wide range of diseases and our experience with managing cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) associated with chimeric antigen receptor (CAR) T-cell therapy, we work with all the departments, including the Department of Pharmacy and the Department of Nursing, to recognize the occurrence of irAEs, CRS, and ICANS and implement countermeasures. We also assist in irAE-related research.
2. Infectious diseases
The mission of the infectious diseases section is to provide consulting on clinical infectious diseases. We also work with the Office of Infection Control and Prevention to prevent healthcare-associated infections during cancer care.
The Team and What We Do
1. General internal medicine
- Increased number of reported irAEs, CRS, and ICANS (Figure 1)
- Implementation of irAE collaboration seminar
- Development of a manual for immune checkpoint inhibitors
Figure1. Reported Number of irAE/CRS/ICANS Cases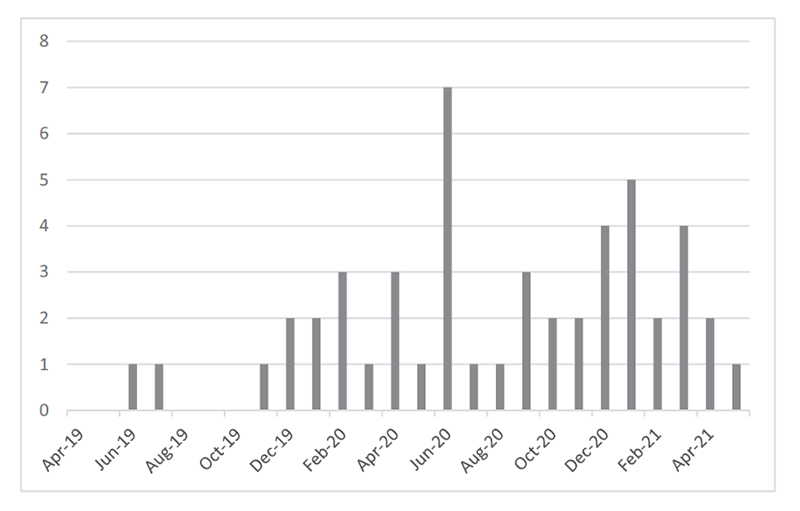

2. Infectious diseases
We have provided 320 infectious disease consultations during this period (Table 1) and have promoted hospital infection control (See the “Office of Infection Control and Prevention” section).
The number of cases managed during this period:
- Positive blood culture cases: 349
- Cases using broad-spectrum antibiotics: 2,402
Table 1. Number of infectious disease consultations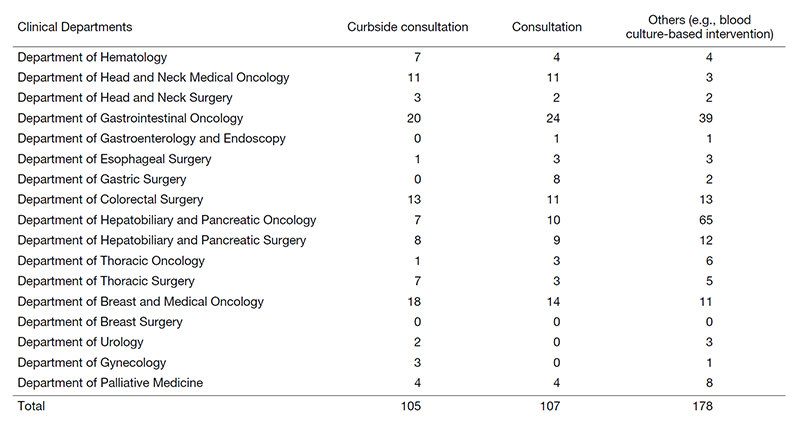

Research activities
- Case presentation support for trainees at the congress of the Japanese Society of Internal Medicine
- Paper writing support
- Intensive lectures on medical oncology for new residents (recorded, updated on the web)
- Support/guidance for new research (e.g., observational study to confirm the pharmacokinetics of mycophenolate mofetil for liver damage caused by immune checkpoint inhibitors)
- Contribution to the training of internal medicine trainees
- irAE cooperation seminar with regional institutions
Clinical trials
One observational study is currently planned by the Department of Pharmacy, a study that we fully support.
Education
We will further collaborate with regional institutions to gather outstanding trainees, and develop close connections with several academic institutions. We will prepare a manual for immune checkpoint inhibitors and develop safe and cost-effective practices. We plan to have approximately 100 case consultations on immune related AEs.
Future Prospects
1. General internal medicine
We will continue to provide high-quality training. The medical treatment for cancer at our hospital is at the top level in Japan, and we will continue to improve the medical treatment system so that the valuable experience gained at this hospital can be returned to general medical treatment, and to establish a consulting system. We will continue the irAE cooperation program and promote research planning for issue identification and improvement. We will deepen our cooperation with the Department of Pharmacy and Department of Nursing, and continue our efforts to share and improve issues within the hospital. Regarding education, we will cooperate with the Human Resource Development Center to enhance internal education and will continuously cooperate with external parties. We will continuously confirm that medical issues can be shared with the risk management department.
2. Infectious diseases
Consultation services for infectious diseases are now increasingly recognized as key components of cancer centers, some of which have begun to establish a department for infectious diseases. The future goal is to launch fellowship programs for fellows to develop high-level expertise and assume a key role in this field.
List of papers published in 2020
Journal
1. Ohmachi K, Kinoshita T, Tobinai K, Ogawa G, Mizutani T, Yamauchi N, Fukuhara N, Uchida T, Yamamoto K, Miyazaki K, Tsukamoto N, Iida S, Utsumi T, Yoshida I, Imaizumi Y, Tokunaga T, Yoshida S, Masaki Y, Murayama T, Yakushijin Y, Suehiro Y, Nosaka K, Dobashi N, Kuroda J, Takamatsu Y, Maruyama D, Ando K, Ishizawa K, Ogura M, Yoshino T, Hotta T, Tsukasaki K, Nagai H. A randomized phase 2/3 study of R-CHOP vs CHOP combined with dose-dense rituximab for DLBCL: the JCOG0601 trial. Blood Adv, 5:984-993, 2021
2. Nozaki K, Maruyama D, Maeshima AM, Tajima K, Itami J, Shichijo T, Yuda S, Suzuki T, Toyoda K, Yamauchi N, Makita S, Fukuhara S, Munakata W, Kobayashi Y, Taniguchi H, Izutsu K, Tobinai K. The role of local radiotherapy following rituximab-containing chemotherapy in patients with transformed indolent B-cell lymphoma. Eur J Haematol, 106:213-220, 2021
3. Amano K, Maeda I, Ishiki H, Miura T, Hatano Y, Tsukuura H, Taniyama T, Matsumoto Y, Matsuda Y, Kohara H, Morita T, Mori M. Effects of enteral nutrition and parenteral nutrition on survival in patients with advanced cancer cachexia: Analysis of a multicenter prospective cohort study. Clin Nutr, 40:1168-1175, 2021
4. Doi T, Fujiwara Y, Shitara K, Shimizu T, Yonemori K, Matsubara N, Ohno I, Kogawa T, Naito Y, Leopold L, Munteanu M, Yatsuzuka N, Han SR, Samkari A, Yamamoto N. The safety and tolerability of epacadostat alone and in combination with pembrolizumab in patients with advanced solid tumors: results from a first-in-Japanese phase I study (KEYNOTE-434). Invest New Drugs, 39:152-162, 2021
5. Powderly J, Spira A, Kondo S, Doi T, Luke JJ, Rasco D, Gao B, Tanner M, Cassier PA, Gazzah A, Italiano A, Tosi D, Afar DE, Parikh A, Engelhardt B, Englert S, Lambert SL, Kasichayanula S, Mensing S, Menon R, Vosganian G, Tolcher A. Model Informed Dosing Regimen and Phase I Results of the Anti-PD-1 Antibody Budigalimab (ABBV-181). Clin Transl Sci, 14:277-287, 2021
6. Sunami K, Naito Y, Aimono E, Amano T, Ennishi D, Kage H, Kanai M, Komine K, Koyama T, Maeda T, Morita S, Sakai D, Kohsaka S, Tsuchihara K, Yoshino T. The initial assessment of expert panel performance in core hospitals for cancer genomic medicine in Japan. Int J Clin Oncol, 26:443-449, 2021
7. Naito Y, Aburatani H, Amano T, Baba E, Furukawa T, Hayashida T, Hiyama E, Ikeda S, Kanai M, Kato M, Kinoshita I, Kiyota N, Kohno T, Kohsaka S, Komine K, Matsumura I, Miura Y, Nakamura Y, Natsume A, Nishio K, Oda K, Oda N, Okita N, Oseto K, Sunami K, Takahashi H, Takeda M, Tashiro S, Toyooka S, Ueno H, Yachida S, Yoshino T, Tsuchihara K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int J Clin Oncol, 26:233-283, 2021
8. Hasegawa Y, Matsubara N, Kogawa T, Naito Y, Harano K, Hosono A, Onishi T, Hojo T, Shimokawa M, Mukohara T. Neo-Bioscore in Guiding Post-surgical Therapy in Patients With Triple-negative Breast Cancer Who Received Neoadjuvant Chemotherapy. In Vivo, 35:1041-1049, 2021
9. Minami H, Doi T, Toyoda M, Imamura Y, Kiyota N, Mitsuma A, Shimokata T, Naito Y, Matsubara N, Tajima T, Tokushige K, Ishihara K, Cameron S, Ando Y. Phase I study of the antiprogrammed cell death-1 Ab spartalizumab (PDR001) in Japanese patients with advanced malignancies. Cancer Sci, 112:725-733, 2021
10. Tsurutani J, Hara F, Kitada M, Takahashi M, Kikawa Y, Kato H, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Taira N, Takashima T, Kashiwabara K, Aihara T, Mukai H. Randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast, 55:63-68, 2021
11. Yamamoto M, Yoshida M, Furuse J, Sano K, Ohtsuka M, Yamashita S, Beppu T, Iwashita Y, Wada K, Nakajima TE, Sakamoto K, Hayano K, Mori Y, Asai K, Matsuyama R, Hirashita T, Hibi T, Sakai N, Tabata T, Kawakami H, Takeda H, Mizukami T, Ozaka M, Ueno M, Naito Y, Okano N, Ueno T, Hijioka S, Shikata S, Ukai T, Strasberg S, Sarr MG, Jagannath P, Hwang TL, Han HS, Yoon YS, Wang HJ, Luo SC, Adam R, Gimenez M, Scatton O, Oh DY, Takada T. Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J Hepatobiliary Pancreat Sci, 28:1-25, 2021
12. Minamide T, Ikematsu H, Murano T, Kadota T, Shinmura K, Yoda Y, Hori K, Ito M, Yano T. Metachronous advanced neoplasia after submucosal invasive colorectal cancer resection. Sci Rep, 11:1869, 2021
13. Hatake K, Chou T, Doi T, Terui Y, Kato H, Hirose T, Seo S, Pourdehnad M, Ogaki Y, Fujimoto H, Hagner PR, Yamamoto K. Phase I, multicenter, dose-escalation study of avadomide in adult Japanese patients with advanced malignancies. Cancer Sci, 112:331-338, 2021
14. Kashima Y, Togashi Y, Fukuoka S, Kamada T, Irie T, Suzuki A, Nakamura Y, Shitara K, Minamide T, Yoshida T, Taoka N, Kawase T, Wada T, Inaki K, Chihara M, Ebisuno Y, Tsukamoto S, Fujii R, Ohashi A, Suzuki Y, Tsuchihara K, Nishikawa H, Doi T. Potentiality of multiple modalities for single-cell analyses to evaluate the tumor microenvironment in clinical specimens. Sci Rep, 11:341, 2021
15. Sunakawa H, Hori K, Kadota T, Shinmura K, Yoda Y, Ikematsu H, Tomioka T, Akimoto T, Hayashi R, Fujii S, Yano T. Relationship between the microvascular patterns observed by magnifying endoscopy with narrow-band imaging and the depth of invasion in superficial pharyngeal squamous cell carcinoma. Esophagus, 18:111-117, 2021
16. Nakajo K, Yoda Y, Kadota T, Murano T, Shinmura K, Ikematsu H, Akimoto T, Yano T. Radial incision and cutting for dilation before endoscopic submucosal dissection in patients with esophageal cancer on the distal side of severe benign esophageal strictures. Dis Esophagus, 34:2021
17. Nishihara K, Hori K, Saito T, Omori T, Sunakawa H, Minamide T, Suyama M, Yamamoto Y, Yoda Y, Shinmura K, Ikematsu H, Yano T. A study of evaluating specific tissue oxygen saturation values of gastrointestinal tumors by removing adherent substances in oxygen saturation imaging. PLoS One, 16:e0243165, 2021
18. Ito R, Ikematsu H, Murano T, Shinmura K, Kojima M, Kumahara K, Furue Y, Sunakawa H, Minamide T, Sato D, Yamamoto Y, Takashima K, Yoda Y, Hori K, Yano T. Diagnostic ability of Japan Narrow-Band Imaging Expert Team classification for colorectal lesions by magnifying endoscopy with blue laser imaging versus narrow-band imaging. Endosc Int Open, 9:E271-E277, 2021
19. Ito R, Kadota T, Murano T, Yoda Y, Hori K, Minamide T, Sato D, Yamamoto Y, Takashima K, Shinmura K, Ikematsu H, Yano T. Clinical features and risk factors of gastric cancer detected by esophagogastroduodenoscopy in esophageal cancer patients. Esophagus, 18:621-628, 2021
20. Yamagishi T, Ohnishi M, Matsunaga N, Kakimoto K, Kamiya H, Okamoto K, Suzuki M, Gu Y, Sakaguchi M, Tajima T, Takaya S, Ohmagari N, Takeda M, Matsuyama S, Shirato K, Nao N, Hasegawa H, Kageyama T, Takayama I, Saito S, Wada K, Fujita R, Saito H, Okinaka K, Griffith M, Parry AE, Barnetson B, Leonard J, Wakita T. Corrigendum to: Environmental Sampling for Severe Acute Respiratory Syndrome Coronavirus 2 During a COVID-19 Outbreak on the Diamond Princess Cruise Ship. J Infect Dis, 223:540, 2021
21. Shimoi T, Nagai SE, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T. The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer, 27:322-331, 2020
22. Doi T, Boku N, Onozawa Y, Takahashi K, Kawaguchi O, Ohtsu A. Phase I dose-escalation study of the safety, tolerability, and pharmacokinetics of aflibercept in combination with S-1 in Japanese patients with advanced solid malignancies. Invest New Drugs, 38:1390-1399, 2020
23. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol, 38:4138-4148, 2020
24. Doi T, Matsubara N, Kawai A, Naka N, Takahashi S, Uemura H, Yamamoto N. Phase I study of TAS-115, a novel oral multi-kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs, 38:1175-1185, 2020
25. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol, 21:1346-1358, 2020
26. Onishi A, Inamoto Y, Tajima K, Yamaguchi J, Kawashima I, Kawajiri A, Takemura T, Ito A, Tanaka T, Okinaka K, Fuji S, Kurosawa S, Kim SW, Fukuda T. Detrimental effects of pretransplant cisplatin-based chemotherapy on renal function after allogeneic hematopoietic cell transplantation for lymphoma. Bone Marrow Transplant, 55:2196-2198, 2020
27. Kang YK, Bang YJ, Kondo S, Chung HC, Muro K, Dussault I, Helwig C, Osada M, Doi T. Safety and Tolerability of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Asian Patients with Pretreated Recurrent or Refractory Gastric Cancer. Clin Cancer Res, 26:3202-3210, 2020
28. Kondo S, Tajimi M, Funai T, Inoue K, Asou H, Ranka VK, Wacheck V, Doi T. Phase 1 dose-escalation study of a novel oral PI3K/mTOR dual inhibitor, LY3023414, in patients with cancer. Invest New Drugs, 38:1836-1845, 2020
29. Shingaki S, Kogawa T, Shimokawa M, Harano K, Naito Y, Kusuhara S, Fujimoto Y, Matsubara N, Hosono A, Mukai H, Onishi T, Hojo T, Mukohara T. Use of eribulin as an earlier-line chemotherapy for patients with HER2-negative metastatic breast cancer. J Cancer, 11:4099-4105, 2020
30. Demetri GD, Antonescu CR, Bjerkehagen B, Bov?e JVMG, Boye K, Chac?n M, Dei Tos AP, Desai J, Fletcher JA, Gelderblom H, George S, Gronchi A, Haas RL, Hindi N, Hohenberger P, Joensuu H, Jones RL, Judson I, Kang YK, Kawai A, Lazar AJ, Le Cesne A, Maestro R, Maki RG, Mart?n J, Patel S, Penault-Llorca F, Premanand Raut C, Rutkowski P, Safwat A, Sbaraglia M, Schaefer IM, Shen L, Serrano C, Sch?ffski P, Stacchiotti S, Sundby Hall K, Tap WD, Thomas DM, Trent J, Valverde C, van der Graaf WTA, von Mehren M, Wagner A, Wardelmann E, Naito Y, Zalcberg J, Blay JY. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: expert recommendations from the World Sarcoma Network. Ann Oncol, 31:1506-1517, 2020
31. Shitara K, Yamazaki K, Tsushima T, Naito T, Matsubara N, Watanabe M, Sarholz B, Johne A, Doi T. Phase I trial of the MET inhibitor tepotinib in Japanese patients with solid tumors. Jpn J Clin Oncol, 50:859-866, 2020
32. Yoshino T, Pentheroudakis G, Mishima S, Overman MJ, Yeh KH, Baba E, Naito Y, Calvo F, Saxena A, Chen LT, Takeda M, Cervantes A, Taniguchi H, Yoshida K, Kodera Y, Kitagawa Y, Tabernero J, Burris H, Douillard JY. JSCO-ESMO-ASCO-JSMO-TOS: international expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol, 31:861-872, 2020
33. Sasaki A, Harano K, Kogawa T, Matsubara N, Naito Y, Hosono A, Mukai H, Yoshino T, Mukohara T. Intestinal Perforation due to Neutropenic Enterocolitis in a Patient Treated with Bevacizumab for Ovarian Cancer. Case Rep Oncol Med, 2020:7231358, 2020
34. Kawazoe A, Kuboki Y, Bando H, Fukuoka S, Kojima T, Naito Y, Iino S, Yodo Y, Doi T, Shitara K, Yoshino T. Phase 1 study of napabucasin, a cancer stemness inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol, 85:855-862, 2020
35. Matsumoto K, Takahashi M, Sato K, Osaki A, Takano T, Naito Y, Matsuura K, Aogi K, Fujiwara K, Tamura K, Baba M, Tokunaga S, Hirano G, Imoto S, Miyazaki C, Yanagihara K, Imamura CK, Chiba Y, Saeki T. A double-blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy-induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide. Cancer Med, 9:3319-3327, 2020
36. Tap WD, Wagner AJ, Sch?ffski P, Martin-Broto J, Krarup-Hansen A, Ganjoo KN, Yen CC, Abdul Razak AR, Spira A, Kawai A, Le Cesne A, Van Tine BA, Naito Y, Park SH, Fedenko A, P?pai Z, Soldatenkova V, Shahir A, Mo G, Wright J, Jones RL. Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA, 323:1266-1276, 2020
37. Inoue M, Naito Y, Kogawa T, Kusuhara S, Fukasawa Y, Fukasawa Y, Harano K Matsubara N, Hosono A, Mukohara T. Safety and Efficacy of Palbociclib in Male Metastatic Breast Cancer: A Report of Two Cases. Ann Case Report, 14:416, 2020
38. Ikeda T, Takemoto S, Senju H, Gyotoku H, Taniguchi H, Shimada M, Dotsu Y, Umeyama Y, Tomono H, Kitazaki T, Fukuda M, Soda H, Yamaguchi H, Fukuda M, Mukae H. Amrubicin in previously treated patients with malignant pleural mesothelioma: A phase II study. Thorac Cancer, 11:1972-1978, 2020
39. Doi T, Fujiwara Y, Koyama T, Ikeda M, Helwig C, Watanabe M, Vugmeyster Y, Kudo M. Phase I Study of the Bifunctional Fusion Protein Bintrafusp Alfa in Asian Patients with Advanced Solid Tumors, Including a Hepatocellular Carcinoma Safety-Assessment Cohort. Oncologist, 25:e1292-e1302, 2020
40. Kubota Y, Kawazoe A, Sasaki A, Mishima S, Sawada K, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Ishii G, Kuwata T, Shitara K. The Impact of Molecular Subtype on Efficacy of Chemotherapy and Checkpoint Inhibition in Advanced Gastric Cancer. Clin Cancer Res, 26:3784-3790, 2020
41. Ishii T, Kawazoe A, Sasaki A, Mishima S, Kentaro S, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Kuwata T, Ishii G, Shitara K. Clinical and molecular factors for selection of nivolumab or irinotecan as third-line treatment for advanced gastric cancer. Ther Adv Med Oncol, 12:1758835920942377, 2020
42. Sasaki A, Kawazoe A, Eto T, Okunaka M, Mishima S, Sawada K, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Akimoto T, Shitara K. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer. ESMO Open, 4:2020
43. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, Yuki S, Wakabayashi M, Nomura S, Sato A, Kuwata T, Kawazu M, Mano H, Togashi Y, Nishikawa H, Yoshino T. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin Cancer Res, 26:5887-5894, 2020
44. Sasaki A, Nakamura Y, Togashi Y, Kuno H, Hojo H, Kageyama S, Nakamura N, Takashima K, Kadota T, Yoda Y, Mishima S, Sawada K, Kotani D, Kawazoe A, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Yano T, Kobayashi T, Akimoto T, Nishikawa H, Shitara K. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer, 23:893-903, 2020
45. Nakamura Y, Sasaki A, Yukami H, Jogo T, Kawazoe A, Kuboki Y, Taniguchi H, Yamashita R, Kuwata T, Ozawa M, Nakamura M, Yoshino T, Shitara K. Emergence of Concurrent Multiple EGFR Mutations and MET Amplification in a Patient With EGFR-Amplified Advanced Gastric Cancer Treated With Cetuximab. JCO Precis Oncol, 4:2020
46. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, Rugo HS, de Braud F, Varga AI, Hansen A, Wang H, Krishnan S, Norwood KG, Doi T. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer, 147:2190-2198, 2020
47. Kondo S, Tajimi M, Funai T, Inoue K, Asou H, Ranka VK, Wacheck V, Doi T. Correction to: Phase 1 dose-escalation study of a novel oral PI3K/mTOR dual inhibitor, LY3023414, in patients with cancer. Invest New Drugs, 38:1846, 2020
48. Gelderblom H, Jones RL, George S, Valverde Morales C, Benson C, Jean-Yves Blay, Renouf DJ, Doi T, Le Cesne A, Leahy M, Hertle S, Aimone P, Brandt U, Sch?ffski P. Imatinib in combination with phosphoinositol kinase inhibitor buparlisib in patients with gastrointestinal stromal tumour who failed prior therapy with imatinib and sunitinib: a Phase 1b, multicentre study. Br J Cancer, 122:1158-1165, 2020
49. Tsurutani J, Iwata H, Krop I, J?nne PA, Doi T, Takahashi S, Park H, Redfern C, Tamura K, Wise-Draper TM, Saito K, Sugihara M, Singh J, Jikoh T, Gallant G, Li BT. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov, 10:688-701, 2020
50. Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, Bergsland E, Shah M, Fakih M, Takahashi S, Piha-Paul SA, O'Neil B, Thomas S, Lolkema MP, Chen M, Ibrahim N, Norwood K, Hadoux J. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin Cancer Res, 26:2124-2130, 2020
51. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol, 38:1887-1896, 2020
52. Nishida T, Doi T. A new approach to refractory gastrointestinal stromal tumours with diverse acquired mutations. Lancet Oncol, 21:864-865, 2020
53. Tabernero J, Alsina M, Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, Ghidini M, Faustino C, Gorbunova V, Zhavrid E, Nishikawa K, Ando T, Yal??n ?, Van Cutsem E, Sabater J, Skanji D, Leger C, Amellal N, Ilson DH. Health-related quality of life associated with trifluridine/tipiracil in heavily pretreated metastatic gastric cancer: results from TAGS. Gastric Cancer, 23:689-698, 2020
54. Tsumura R, Koga Y, Hamada A, Kuwata T, Sasaki H, Doi T, Aikawa K, Ohashi A, Katano I, Ikarashi Y, Ito M, Ochiai A. Report of the use of patient-derived xenograft models in the development of anticancer drugs in Japan. Cancer Sci, 111:3386-3394, 2020
55. Sato D, Motegi A, Kadota T, Kojima T, Bando H, Shinmura K, Hori K, Yoda Y, Oono Y, Zenda S, Ikematsu H, Akimoto T, Yano T. Therapeutic results of proton beam therapy with concurrent chemotherapy for cT1 esophageal cancer and salvage endoscopic therapy for local recurrence. Esophagus, 17:305-311, 2020
56. Mehnert JM, Bergsland E, O'Neil BH, Santoro A, Schellens JHM, Cohen RB, Doi T, Ott PA, Pishvaian MJ, Puzanov I, Aung KL, Hsu C, Le Tourneau C, Hollebecque A, ?lez E, Tamura K, Gould M, Yang P, Stein K, Piha-Paul SA. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer, 126:3021-3030, 2020
57. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med, 383:1207-1217, 2020
58. Murano T, Ikematsu H, Shinmura K, Ito R, Minamide T, Sato D, Yamamoto Y, Takashima K, Kadota T, Yoda Y, Hori K, Oono Y, Yano T. Endoscopic prediction of advanced histology in colorectal lesions sized <10 mm using the Japan Narrow-band imaging Expert Team classification. Dig Endosc, 32:785-790, 2020
59. Kadota T, Yoda Y, Hori K, Shinmura K, Oono Y, Ikematsu H, Yano T. Prophylactic steroid administration against strictures is not enough for mucosal defects involving the entire circumference of the esophageal lumen after esophageal endoscopic submucosal dissection (ESD). Esophagus, 17:440-447, 2020
60. Watanabe K, Mitsunaga S, Kojima M, Suzuki H, Irisawa A, Takahashi H, Sasaki M, Hashimoto Y, Imaoka H, Ohno I, Ikeda M, Akimoto T, Ochiai A. The "histological replacement growth pattern" represents aggressive invasive behavior in liver metastasis from pancreatic cancer. Cancer Med, 9:3130-3141, 2020
61. Kan M, Imaoka H, Watanabe K, Sasaki M, Takahashi H, Hashimoto Y, Ohno I, Mitsunaga S, Umemoto K, Kimura G, Suzuki Y, Eguchi H, Otsuru T, Goda K, Ikeda M. Chemotherapy-induced neutropenia as a prognostic factor in patients with pancreatic cancer treated with gemcitabine plus nab-paclitaxel: a retrospective cohort study. Cancer Chemother Pharmacol, 86:203-210, 2020
62. Kuzume A, Chi S, Yamauchi N, Minami Y. Immune-Checkpoint Blockade Therapy in Lymphoma. Int J Mol Sci, 21:2020
63. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Reply to T.J.A. Dekker. J Clin Oncol, 38:3351-3352, 2020
64. Tagami K, Kawaguchi T, Miura T, Yamaguchi T, Matsumoto Y, Watanabe YS, Uehara Y, Okizaki A, Inoue A, Morita T, Kinoshita H. The association between health-related quality of life and achievement of personalized symptom goal. Support Care Cancer, 28:4737-4743, 2020
65. Nagasaki J, Togashi Y, Sugawara T, Itami M, Yamauchi N, Yuda J, Sugano M, Ohara Y, Minami Y, Nakamae H, Hino M, Takeuchi M, Nishikawa H. The critical role of CD4+ T cells in PD-1 blockade against MHC-II-expressing tumors such as classic Hodgkin lymphoma. Blood Adv, 4:4069-4082, 2020
66. Yamagishi T, Ohnishi M, Matsunaga N, Kakimoto K, Kamiya H, Okamoto K, Suzuki M, Gu Y, Sakaguchi M, Tajima T, Takaya S, Ohmagari N, Takeda M, Matsuyama S, Shirato K, Nao N, Hasegawa H, Kageyama T, Takayama I, Saito S, Wada K, Fujita R, Saito H, Okinaka K, Griffith M, Parry AE, Barnetson B, Leonard J, Wakita T. Environmental Sampling for Severe Acute Respiratory Syndrome Coronavirus 2 During a COVID-19 Outbreak on the Diamond Princess Cruise Ship. J Infect Dis, 222:1098-1102, 2020
67. Shichijo T, Maruyama D, Yamauchi N, Maeshima AM, Sugano M, Yuda S, Tajima K, Kurihara H, Shimada K, Suzuki T, Toyoda K, Makita S, Fukuhara S, Munakata W, Suzuki T, Kobayashi Y, Taniguchi H, Minami Y, Izutsu K, Tobinai K. Transformation Scoring System (TSS): A new assessment index for clinical transformation of follicular lymphoma. Cancer Med, 9:8864-8874, 2020
68. Yamashita C, Takesue Y, Matsumoto K, Ikegame K, Enoki Y, Uchino M, Miyazaki T, Izumikawa K, Takada T, Okinaka K, Ueda T, Miyazaki Y, Mayumi T. Echinocandins versus non-echinocandins for empirical antifungal therapy in patients with hematological disease with febrile neutropenia: A systematic review and meta-analysis. J Infect Chemother, 26:596-603, 2020
