Annual Report 2020
Department of Palliative Medicine
Yoshihisa Matsumoto, Tomofumi Miura, Kazuhiro Kosugi, Tatsuto Terada, Yajima Yuki Midori, Maika Natsume, Yasutaka Shimotsuura, Emi Kubo, Misao Fukuda, Yuko Uehara, Yukako Hattori, Rie Yamamoto, Masako Ikeda, Sachiko Nagatsuma
Introduction
The purpose of our department is to improve the quality of life of cancer patients and their family caregivers by managing irritable symptom burden and establishing a regional palliative care system. For that purpose, we provide 3 palliative care services: (1) outpatient clinic, (2) palliative care unit, and (3) supportive care team.
Additionally, the National Cancer Center Hospital East was certified as an ESMO designated center of integrated oncology and palliative care in 2018.
The Team and What We Do
1. Outpatient clinic
Patients with or without anti-cancer therapy consult our outpatient clinic about management of their symptoms or support to decide where and how to spend their lives (Table 1).
2. Palliative care unit
Our palliative care unit is the Japanese version of an acute palliative care unit (APCU). The features of the APCU are multidimensional assessment, rapid symptom control, and intensive psychosocial care with a shorter length of stay and lower death rate than in a traditional PCU. Medical social workers greatly contribute to the transition to palliative home care and transfers to other hospitals (Table 2).
3. Supportive care team
This team consists of physicians, psycho-oncologists, nurses, dieticians, physiotherapists, and speech-language-hearing therapists. In the oncology section, our supportive care team takes a multidisciplinary approach for inpatients with various illnesses (Table 3).
Table 1. Number of patients in outpatients clinic 

Table 2. Number of patients in the PCU

Table 3. Number of patients treated by supportive care team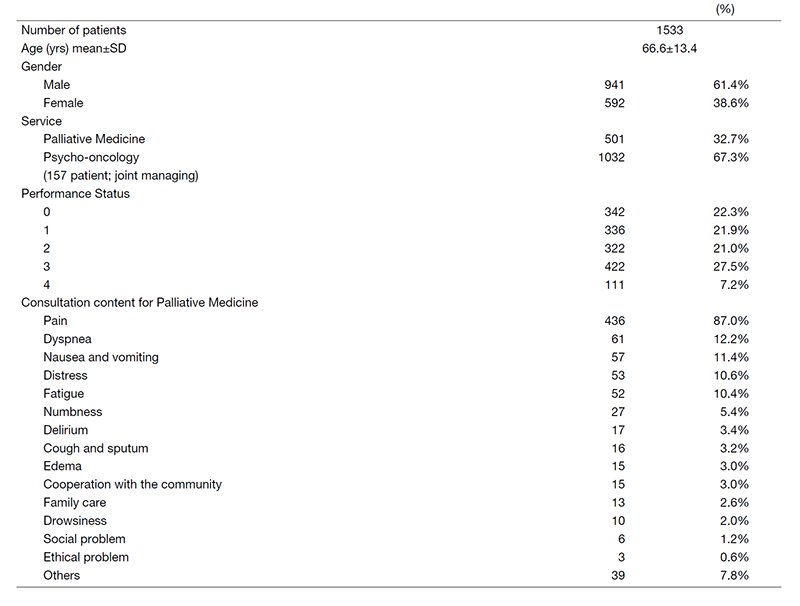

Research activities
1) Development of the integration of early palliative care in metastatic lung cancer.
2) Development of an intervention for cancer-related fatigue in advanced cancer patients in palliative care settings.
3) Development of a support system for cancer patients at home using AI-based monitoring.
4) Research on the establishment of a support system for cancer patients with minor children using an online peer support system.
5) Survey on the perceptions and psychological burden of hematologists and palliative care physicians regarding end-stage blood transfusion therapy.
6) Nationwide survey on refractory cancer pain and symptoms.
7) Research on the actual status of cancer pain treatment for cancer patients who are using specialized palliative care services.
8) Research on the efficacy and safety of systemic administration of steroids for neuropathic pain in cancer patients.
9) Research on the efficacy of specialized rehabilitation for cancer patients hospitalized in palliative care units.
10) Participation in multicenter observational studies, interventional studies, and surveys of bereaved families.
Clinical trials
1) A multicenter randomized controlled trial of the integration of early palliative care in metastatic lung cancer.
2) A multicenter phase II trial of dexamethasone for cancer-related fatigue.
Education
The purpose is to promote our residents’ understanding of palliative care for cancer patients and their families. Residents can train in home palliative care on request. To disseminate knowledge about primary palliative care, we held several lectures for medical staff in NCCHE. Due to the COVID-19 pandemic, we did not hold the workshop which we had planned for regional palliative care staff.
Future Prospects
Our department will continue the above activities and develop new treatments or medical instruments to improve QOL in cancer patients and their family caregivers.
List of papers published in 2020
Journal
1. Amano K, Maeda I, Ishiki H, Miura T, Hatano Y, Tsukuura H, Taniyama T, Matsumoto Y, Matsuda Y, Kohara H, Morita T, Mori M. Effects of enteral nutrition and parenteral nutrition on survival in patients with advanced cancer cachexia: Analysis of a multicenter prospective cohort study. Clin Nutr, 40:1168-1175, 2021
2. Fujisawa D, Umemura S, Okizaki A, Satomi E, Yamaguchi T, Miyaji T, Mashiko T, Kobayashi N, Kinoshita H, Mori M, Morita T, Uchitomi Y, Goto K, Ohe Y, Matsumoto Y. Nurse-led, screening-triggered, early specialised palliative care intervention programme for patients with advanced lung cancer: study protocol for a multicentre randomised controlled trial. BMJ Open, 10:e037759, 2020
3. Matsuoka H, Iwase S, Miyaji T, Kawaguchi T, Ariyoshi K, Oyamada S, Satomi E, Ishiki H, Hasuo H, Sakuma H, Tokoro A, Matsuda Y, Tahara K, Otani H, Ohtake Y, Tsukuura H, Matsumoto Y, Hasegawa Y, Kataoka Y, Otsuka M, Sakai K, Nakura M, Morita T, Yamaguchi T, Koyama A. Predictors of duloxetine response in patients with neuropathic cancer pain: a secondary analysis of a randomized controlled trial-JORTC-PAL08 (DIRECT) study. Support Care Cancer, 28:2931-2939, 2020
4. Maeda I, Ogawa A, Yoshiuchi K, Akechi T, Morita T, Oyamada S, Yamaguchi T, Imai K, Sakashita A, Matsumoto Y, Uemura K, Nakahara R, Iwase S. Safety and effectiveness of antipsychotic medication for delirium in patients with advanced cancer: A large-scale multicenter prospective observational study in real-world palliative care settings. Gen Hosp Psychiatry, 67:35-41, 2020
5. Tagami K, Kawaguchi T, Miura T, Yamaguchi T, Matsumoto Y, Watanabe YS, Uehara Y, Okizaki A, Inoue A, Morita T, Kinoshita H. The association between health-related quality of life and achievement of personalized symptom goal. Support Care Cancer, 28:4737-4743, 2020
6. Mori M, Morita T, Matsuda Y, Yamada H, Kaneishi K, Matsumoto Y, Matsuo N, Odagiri T, Aruga E, Watanabe H, Tatara R, Sakurai H, Kimura A, Katayama H, Suga A, Nishi T, Shirado AN, Watanabe T, Kuchiba A, Yamaguchi T, Iwase S. How successful are we in relieving terminal dyspnea in cancer patients? A real-world multicenter prospective observational study. Support Care Cancer, 28:3051-3060, 2020
7. Usui Y, Ishiki H, Shimomura A, Satomi E. Suggestions Regarding the GEICAM/2003-11_CIBOMA/2004-01 Trial: Future Treatment Options for Early Triple-Negative Breast Cancer. J Clin Oncol, 38:2111-2112, 2020
