Annual Report 2020
Department of Experimental Therapeutics
Toshihiko Doi, Yasutoshi Kuboki, Kiyotaka Yoh, Yoichi Naito, Kohei Shitara, Kenichi Harano, Junichiro Yuda, Nobuhiko Yamauchi, Shigehiro Koganemaru, Takehiro Nakao
Introduction
The NCC-EPOC Phase I Group was organized to promote early drug development, especially the first in human (FIH) trial, and in 2012 the phase I group consisted of two subunits (NCCE-Kashiwa & NCC-Tsukiji) organized by each hospital. The goal of each unit is to perform initial clinical evaluations of promising new anti-cancer compounds emerging from the laboratory. Our Phase 1 unit is the largest program in Japan, indeed in Asia, and we contribute to the development of new cancer drugs through early phase trials.
In April 2013, the Department of Experimental Therapeutics was launched to strongly promote the EPOC missions as previously described. The members of the Department of Experimental Therapeutics consisted of specialists in their oncology fields. We have also conducted/contributed to IIT using unapproved drugs and new academic seeds.
The Team and What We Do
Our team has conducted and managed early drug development, especially the first in human (FIH) trial.
Research activities
This department plays an important role in new anti-cancer drug development in our center as well as in the rest of Japan. Our top priority is to conduct the FIH trials, as well as performing the phase I trials. Recently, we joined the global phase I trial to accelerate new drug development in Japan. Web or tele-conferences are held with the EU and US sites, and we are discussing patient enrollment as well as further developmental strategies. Routine web conferences are also held between the Kashiwa and Tsukiji campuses every Friday morning; we share information about adverse events and patient enrollment and refer the candidates to each other to accelerate enrollment. Several IIT-FIHs using new class seeds and also unapproved company agents are conducted by each unit.
Clinical trials
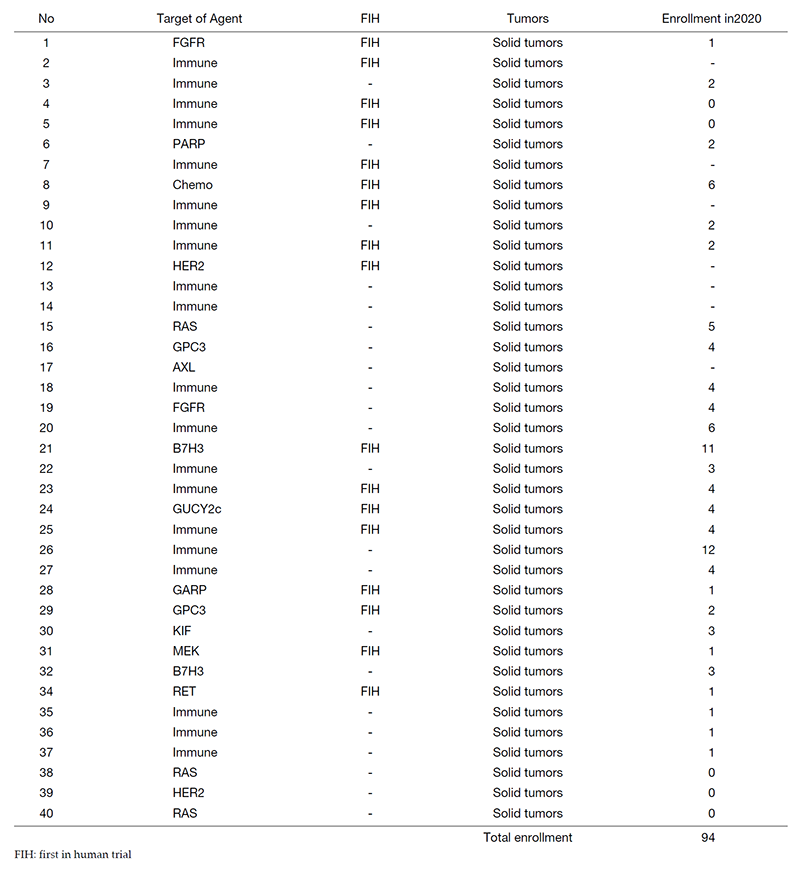
In 2020, 40 phase I trials were conducted. (Table 1).
List of papers published in 2020
Journal
1. Tanimoto A, Matsumoto S, Takeuchi S, Arai S, Fukuda K, Nishiyama A, Yoh K, Ikeda T, Furuya N, Nishino K, Ohe Y, Goto K, Yano S. Proteasome Inhibition Overcomes ALK-TKI Resistance in ALK-Rearranged/TP53-Mutant NSCLC via Noxa Expression. Clin Cancer Res, 27:1410-1420, 2021
2. Kawazoe A, Shitara K, Boku N, Yoshikawa T, Terashima M. Current status of immunotherapy for advanced gastric cancer. Jpn J Clin Oncol, 51:20-27, 2021
3. Takahashi H, Ikeda M, Shiba S, Imaoka H, Todaka A, Shioji K, Yane K, Kojima Y, Kobayashi S, Asagi A, Ozaka M, Takada R, Nagashio Y, Horiguchi S, Kasuga A, Suzuki E, Terashima T, Ueno M, Morizane C, Furuse J. Multicenter Retrospective Analysis of Chemotherapy for Advanced Pancreatic Acinar Cell Carcinoma: Potential Efficacy of Platinum- and Irinotecan-Containing Regimens. Pancreas, 50:77-82, 2021
4. Umemoto K, Takahashi H, Morizane C, Yamada I, Shimizu S, Shioji K, Yoshida Y, Motoya M, Mizuno N, Kojima Y, Terashima T, Uesugi K, Ueno M, Furuse J, Akimoto T, Ikeda M. FOLFIRINOX in advanced pancreatic cancer patients with the double-variant type of UGT1A1 *28 and *6 polymorphism: a multicenter, retrospective study. Cancer Chemother Pharmacol, 87:397-404, 2021
5. Ohmachi K, Kinoshita T, Tobinai K, Ogawa G, Mizutani T, Yamauchi N, Fukuhara N, Uchida T, Yamamoto K, Miyazaki K, Tsukamoto N, Iida S, Utsumi T, Yoshida I, Imaizumi Y, Tokunaga T, Yoshida S, Masaki Y, Murayama T, Yakushijin Y, Suehiro Y, Nosaka K, Dobashi N, Kuroda J, Takamatsu Y, Maruyama D, Ando K, Ishizawa K, Ogura M, Yoshino T, Hotta T, Tsukasaki K, Nagai H. A randomized phase 2/3 study of R-CHOP vs CHOP combined with dose-dense rituximab for DLBCL: the JCOG0601 trial. Blood Adv, 5:984-993, 2021
6. Nozaki K, Maruyama D, Maeshima AM, Tajima K, Itami J, Shichijo T, Yuda S, Suzuki T, Toyoda K, Yamauchi N, Makita S, Fukuhara S, Munakata W, Kobayashi Y, Taniguchi H, Izutsu K, Tobinai K. The role of local radiotherapy following rituximab-containing chemotherapy in patients with transformed indolent B-cell lymphoma. Eur J Haematol, 106:213-220, 2021
7. Doi T, Fujiwara Y, Shitara K, Shimizu T, Yonemori K, Matsubara N, Ohno I, Kogawa T, Naito Y, Leopold L, Munteanu M, Yatsuzuka N, Han SR, Samkari A, Yamamoto N. The safety and tolerability of epacadostat alone and in combination with pembrolizumab in patients with advanced solid tumors: results from a first-in-Japanese phase I study (KEYNOTE-434). Invest New Drugs, 39:152-162, 2021
8. Powderly J, Spira A, Kondo S, Doi T, Luke JJ, Rasco D, Gao B, Tanner M, Cassier PA, Gazzah A, Italiano A, Tosi D, Afar DE, Parikh A, Engelhardt B, Englert S, Lambert SL, Kasichayanula S, Mensing S, Menon R, Vosganian G, Tolcher A. Model Informed Dosing Regimen and Phase I Results of the Anti-PD-1 Antibody Budigalimab (ABBV-181). Clin Transl Sci, 14:277-287, 2021
9. Sunami K, Naito Y, Aimono E, Amano T, Ennishi D, Kage H, Kanai M, Komine K, Koyama T, Maeda T, Morita S, Sakai D, Kohsaka S, Tsuchihara K, Yoshino T. The initial assessment of expert panel performance in core hospitals for cancer genomic medicine in Japan. Int J Clin Oncol, 26:443-449, 2021
10. Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Nosaki K, Kohno T, Tsuta K, Nomura S, Ikeno T, Wakabayashi M, Sato A, Matsumoto S, Goto K. Final survival results for the LURET phase II study of vandetanib in previously treated patients with RET-rearranged advanced non-small cell lung cancer. Lung Cancer, 155:40-45, 2021
11. Naito Y, Aburatani H, Amano T, Baba E, Furukawa T, Hayashida T, Hiyama E, Ikeda S, Kanai M, Kato M, Kinoshita I, Kiyota N, Kohno T, Kohsaka S, Komine K, Matsumura I, Miura Y, Nakamura Y, Natsume A, Nishio K, Oda K, Oda N, Okita N, Oseto K, Sunami K, Takahashi H, Takeda M, Tashiro S, Toyooka S, Ueno H, Yachida S, Yoshino T, Tsuchihara K. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (edition 2.1). Int J Clin Oncol, 26:233-283, 2021
12. Hasegawa Y, Matsubara N, Kogawa T, Naito Y, Harano K, Hosono A, Onishi T, Hojo T, Shimokawa M, Mukohara T. Neo-Bioscore in Guiding Post-surgical Therapy in Patients With Triple-negative Breast Cancer Who Received Neoadjuvant Chemotherapy. In Vivo, 35:1041-1049, 2021
13. Okamoto A, Kondo E, Nakamura T, Yanagida S, Hamanishi J, Harano K, Hasegawa K, Hirasawa T, Hori K, Komiyama S, Matsuura M, Nakai H, Nakamura H, Sakata J, Tabata T, Takehara K, Takekuma M, Yokoyama Y, Kase Y, Sumino S, Soeda J, Suri A, Aoki D, Sugiyama T. Phase 2 single-arm study on the efficacy and safety of niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer. J Gynecol Oncol, 32:e16, 2021
14. Minami H, Doi T, Toyoda M, Imamura Y, Kiyota N, Mitsuma A, Shimokata T, Naito Y, Matsubara N, Tajima T, Tokushige K, Ishihara K, Cameron S, Ando Y. Phase I study of the antiprogrammed cell death-1 Ab spartalizumab (PDR001) in Japanese patients with advanced malignancies. Cancer Sci, 112:725-733, 2021
15. Tsurutani J, Hara F, Kitada M, Takahashi M, Kikawa Y, Kato H, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Taira N, Takashima T, Kashiwabara K, Aihara T, Mukai H. Randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast, 55:63-68, 2021
16. Yamamoto M, Yoshida M, Furuse J, Sano K, Ohtsuka M, Yamashita S, Beppu T, Iwashita Y, Wada K, Nakajima TE, Sakamoto K, Hayano K, Mori Y, Asai K, Matsuyama R, Hirashita T, Hibi T, Sakai N, Tabata T, Kawakami H, Takeda H, Mizukami T, Ozaka M, Ueno M, Naito Y, Okano N, Ueno T, Hijioka S, Shikata S, Ukai T, Strasberg S, Sarr MG, Jagannath P, Hwang TL, Han HS, Yoon YS, Wang HJ, Luo SC, Adam R, Gimenez M, Scatton O, Oh DY, Takada T. Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J Hepatobiliary Pancreat Sci, 28:1-25, 2021
17. Perets R, Bar J, Rasco DW, Ahn MJ, Yoh K, Kim DW, Nagrial A, Satouchi M, Lee DH, Spigel DR, Kotasek D, Gutierrez M, Niu J, Siddiqi S, Li X, Cyrus J, Chackerian A, Chain A, Altura RA, Cho BC. Safety and efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in combination with pembrolizumab in first-line advanced non-small-cell lung cancer. Ann Oncol, 32:395-403, 2021
18. Shibata Y, Matsumoto S, Yoh K, Goto K. Evaluation of variant frequency in SARS-CoV-2 infection-related genes utilizing lung cancer genomic database. Lung Cancer, 152:199-201, 2021
19. Takeuchi S, Yanagitani N, Seto T, Hattori Y, Ohashi K, Morise M, Matsumoto S, Yoh K, Goto K, Nishio M, Takahara S, Kawakami T, Imai Y, Yoshimura K, Tanimoto A, Nishiyama A, Murayama T, Yano S. Phase 1/2 study of alectinib in RET-rearranged previously-treated non-small cell lung cancer (ALL-RET). Transl Lung Cancer Res, 10:314-325, 2021
20. Koganemaru S, Shitara K. Antibody-drug conjugates to treat gastric cancer. Expert Opin Biol Ther, 21:923-930, 2021
21. Hatake K, Chou T, Doi T, Terui Y, Kato H, Hirose T, Seo S, Pourdehnad M, Ogaki Y, Fujimoto H, Hagner PR, Yamamoto K. Phase I, multicenter, dose-escalation study of avadomide in adult Japanese patients with advanced malignancies. Cancer Sci, 112:331-338, 2021
22. Kotani D, Shitara K. Trastuzumab deruxtecan for the treatment of patients with HER2-positive gastric cancer. Ther Adv Med Oncol, 13:1758835920986518, 2021
23. Kashima Y, Togashi Y, Fukuoka S, Kamada T, Irie T, Suzuki A, Nakamura Y, Shitara K, Minamide T, Yoshida T, Taoka N, Kawase T, Wada T, Inaki K, Chihara M, Ebisuno Y, Tsukamoto S, Fujii R, Ohashi A, Suzuki Y, Tsuchihara K, Nishikawa H, Doi T. Potentiality of multiple modalities for single-cell analyses to evaluate the tumor microenvironment in clinical specimens. Sci Rep, 11:341, 2021
24. Fujitani K, Shitara K, Takashima A, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Kimura Y, Amagai K, Hosaka H, Komatsu Y, Shimada K, Kawabata R, Ohdan H, Kodera Y, Nakamura M, Nakajima TE, Miyata Y, Moriwaki T, Kusumoto T, Nishikawa K, Ogata K, Shimura M, Morita S, Koizumi W. Effect of early tumor response on the health-related quality of life among patients on second-line chemotherapy for advanced gastric cancer in the ABSOLUTE trial. Gastric Cancer, 24:467-476, 2021
25. Fujitani K, Shitara K, Takashima A, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Kimura Y, Amagai K, Hosaka H, Komatsu Y, Shimada K, Kawabata R, Ohdan H, Kodera Y, Nakamura M, Nakajima TE, Miyata Y, Moriwaki T, Kusumoto T, Nishikawa K, Ogata K, Shimura M, Morita S, Koizumi W. Correction to: Effect of early tumor response on the health-related quality of life among patients on second-line chemotherapy for advanced gastric cancer in the ABSOLUTE trial. Gastric Cancer, 24:477-478, 2021
26. Yuda J, Yamauchi N, Kuzume A, Guo YM, Sato N, Minami Y. Molecular remission after combination therapy with blinatumomab and ponatinib with relapsed/refractory Philadelphia chromosome-positive acute lymphocytic leukemia: two case reports. J Med Case Rep, 15:164, 2021
27. Kawazoe A, Takahari D, Keisho C, Nakamura Y, Ikeno T, Wakabayashi M, Nomura S, Tamura H, Fukutani M, Hirano N, Saito Y, Kambe M, Sato A, Shitara K. A multicenter phase II study of TAS-114 in combination with S-1 in patients with pretreated advanced gastric cancer (EPOC1604). Gastric Cancer, 24:190-196, 2021
28. Kawazoe A, Ando T, Hosaka H, Fujita J, Koeda K, Nishikawa K, Amagai K, Fujitani K, Ogata K, Watanabe K, Yamamoto Y, Shitara K. Safety and activity of trifluridine/tipiracil and ramucirumab in previously treated advanced gastric cancer: an open-label, single-arm, phase 2 trial. Lancet Gastroenterol Hepatol, 6:209-217, 2021
29. Shimoi T, Nagai SE, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T. The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer, 27:322-331, 2020
30. Kadota T, Ikematsu H, Sasaki T, Saito Y, Ito M, Mizutani T, Ogawa G, Shitara K, Ito Y, Kushima R, Kanemitsu Y, Muto M. Protocol for a single-arm confirmatory trial of adjuvant chemoradiation for patients with high-risk rectal submucosal invasive cancer after local resection: Japan Clinical Oncology Group Study JCOG1612 (RESCUE study). BMJ Open, 10:e034947, 2020
31. Nakajima TE, Yamaguchi K, Boku N, Hyodo I, Mizusawa J, Hara H, Nishina T, Sakamoto T, Shitara K, Shinozaki K, Katayama H, Nakamura S, Muro K, Terashima M. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer, 23:677-688, 2020
32. Doi T, Boku N, Onozawa Y, Takahashi K, Kawaguchi O, Ohtsu A. Phase I dose-escalation study of the safety, tolerability, and pharmacokinetics of aflibercept in combination with S-1 in Japanese patients with advanced solid malignancies. Invest New Drugs, 38:1390-1399, 2020
33. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol, 38:4138-4148, 2020
34. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med, 382:2419-2430, 2020
35. Okano N, Morizane C, Nomura S, Takahashi H, Tsumura H, Satake H, Mizuno N, Tsuji K, Shioji K, Asagi A, Yasui K, Kitagawa S, Kashiwada T, Ishiguro A, Kanai M, Ueno M, Ogura T, Shimizu S, Tobimatsu K, Motoya M, Nakashima K, Ikeda M, Okusaka T, Furuse J. Phase II clinical trial of gemcitabine plus oxaliplatin in patients with metastatic pancreatic adenocarcinoma with a family history of pancreatic/breast/ovarian/prostate cancer or personal history of breast/ovarian/prostate cancer (FABRIC study). Int J Clin Oncol, 25:1835-1843, 2020
36. Doi T, Matsubara N, Kawai A, Naka N, Takahashi S, Uemura H, Yamamoto N. Phase I study of TAS-115, a novel oral multi-kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs, 38:1175-1185, 2020
37. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol, 21:1346-1358, 2020
38. Kang YK, Bang YJ, Kondo S, Chung HC, Muro K, Dussault I, Helwig C, Osada M, Doi T. Safety and Tolerability of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFβ and PD-L1, in Asian Patients with Pretreated Recurrent or Refractory Gastric Cancer. Clin Cancer Res, 26:3202-3210, 2020
39. Kondo S, Tajimi M, Funai T, Inoue K, Asou H, Ranka VK, Wacheck V, Doi T. Phase 1 dose-escalation study of a novel oral PI3K/mTOR dual inhibitor, LY3023414, in patients with cancer. Invest New Drugs, 38:1836-1845, 2020
40. Shingaki S, Kogawa T, Shimokawa M, Harano K, Naito Y, Kusuhara S, Fujimoto Y, Matsubara N, Hosono A, Mukai H, Onishi T, Hojo T, Mukohara T. Use of eribulin as an earlier-line chemotherapy for patients with HER2-negative metastatic breast cancer. J Cancer, 11:4099-4105, 2020
41. Demetri GD, Antonescu CR, Bjerkehagen B, Bov?e JVMG, Boye K, Chac?n M, Dei Tos AP, Desai J, Fletcher JA, Gelderblom H, George S, Gronchi A, Haas RL, Hindi N, Hohenberger P, Joensuu H, Jones RL, Judson I, Kang YK, Kawai A, Lazar AJ, Le Cesne A, Maestro R, Maki RG, Mart?n J, Patel S, Penault-Llorca F, Premanand Raut C, Rutkowski P, Safwat A, Sbaraglia M, Schaefer IM, Shen L, Serrano C, Sch?ffski P, Stacchiotti S, Sundby Hall K, Tap WD, Thomas DM, Trent J, Valverde C, van der Graaf WTA, von Mehren M, Wagner A, Wardelmann E, Naito Y, Zalcberg J, Blay JY. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: expert recommendations from the World Sarcoma Network. Ann Oncol, 31:1506-1517, 2020
42. Shitara K, Yamazaki K, Tsushima T, Naito T, Matsubara N, Watanabe M, Sarholz B, Johne A, Doi T. Phase I trial of the MET inhibitor tepotinib in Japanese patients with solid tumors. Jpn J Clin Oncol, 50:859-866, 2020
43. Yoshino T, Pentheroudakis G, Mishima S, Overman MJ, Yeh KH, Baba E, Naito Y, Calvo F, Saxena A, Chen LT, Takeda M, Cervantes A, Taniguchi H, Yoshida K, Kodera Y, Kitagawa Y, Tabernero J, Burris H, Douillard JY. JSCO-ESMO-ASCO-JSMO-TOS: international expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol, 31:861-872, 2020
44. Sasaki A, Harano K, Kogawa T, Matsubara N, Naito Y, Hosono A, Mukai H, Yoshino T, Mukohara T. Intestinal Perforation due to Neutropenic Enterocolitis in a Patient Treated with Bevacizumab for Ovarian Cancer. Case Rep Oncol Med, 2020:7231358, 2020
45. Iwase T, Harano K, Masuda H, Kida K, Hess KR, Wang Y, Dirix L, Van Laere SJ, Lucci A, Krishnamurthy S, Woodward WA, Layman RM, Bertucci F, Ueno NT. Quantitative hormone receptor (HR) expression and gene expression analysis in HR+ inflammatory breast cancer (IBC) vs non-IBC. BMC Cancer, 20:430, 2020
46. Kawazoe A, Kuboki Y, Bando H, Fukuoka S, Kojima T, Naito Y, Iino S, Yodo Y, Doi T, Shitara K, Yoshino T. Phase 1 study of napabucasin, a cancer stemness inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol, 85:855-862, 2020
47. Matsumoto K, Takahashi M, Sato K, Osaki A, Takano T, Naito Y, Matsuura K, Aogi K, Fujiwara K, Tamura K, Baba M, Tokunaga S, Hirano G, Imoto S, Miyazaki C, Yanagihara K, Imamura CK, Chiba Y, Saeki T. A double-blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy-induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide. Cancer Med, 9:3319-3327, 2020
48. Takehara K, Yamashita N, Watanabe R, Teramoto N, Tsuda H, Motohashi T, Harano K, Nakanishi T, Tokunaga H, Susumu N, Ueda Y, Yokoyama Y, Saito T. Clinical status and prognostic factors in Japanese patients with uterine leiomyosarcoma. Gynecol Oncol, 157:115-120, 2020
49. Tap WD, Wagner AJ, Sch?ffski P, Martin-Broto J, Krarup-Hansen A, Ganjoo KN, Yen CC, Abdul Razak AR, Spira A, Kawai A, Le Cesne A, Van Tine BA, Naito Y, Park SH, Fedenko A, P?pai Z, Soldatenkova V, Shahir A, Mo G, Wright J, Jones RL. Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA, 323:1266-1276, 2020
50. Nishio M, Seto T, Reck M, Garon EB, Chiu CH, Yoh K, Imamura F, Park K, Shih JY, Visseren-Grul C, Frimodt-Moller B, Zimmermann A, Homma G, Enatsu S, Nakagawa K. Ramucirumab or placebo plus erlotinib in EGFR-mutated, metastatic non-small-cell lung cancer: East Asian subset of RELAY. Cancer Sci, 111:4510-4525, 2020
51. Yamamoto N, Hayashi H, Planchard D, Morán T, Gregorc V, Dowell J, Sakai H, Yoh K, Nishio M, Cortot AB, Benhadji KA, Soni N, Huang J, Makris L, Cedres S. A randomized, phase 2 study of deoxyuridine triphosphatase inhibitor, TAS-114, in combination with S-1 versus S-1 alone in patients with advanced non-small-cell lung cancer. Invest New Drugs, 38:1588-1597, 2020
52. Yoh K, Atagi S, Reck M, Garon EB, Ponce Aix S, Moro-Sibilot D, Winfree KB, Frimodt-Moller B, Zimmermann A, Visseren-Grul C, Nakagawa K. Patient-reported outcomes in RELAY, a phase 3 trial of ramucirumab plus erlotinib versus placebo plus erlotinib in untreated EGFR-mutated metastatic non-small-cell lung cancer. Curr Med Res Opin, 36:1667-1675, 2020
53. Nakamura M, Kageyama SI, Udagawa H, Zenke Y, Yoh K, Niho S, Hojo H, Motegi A, Kirita K, Matsumoto S, Goto K, Akimoto T. Differences in failure patterns according to the EGFR mutation status after proton beam therapy for early stage non-small cell lung cancer. Radiother Oncol, 149:14-17, 2020
54. Udagawa H, Kirita K, Naito T, Nomura S, Ishibashi M, Matsuzawa R, Hisakane K, Usui Y, Matsumoto S, Yoh K, Niho S, Ishii G, Goto K. Feasibility and utility of transbronchial cryobiopsy in precision medicine for lung cancer: Prospective single-arm study. Cancer Sci, 111:2488-2498, 2020
55. Yoh K, Takamochi K, Shukuya T, Hishida T, Tsuboi M, Sakurai H, Goto Y, Yoshida K, Ohde Y, Okumura S, Ohashi Y, Kunitoh H. Corrigendum to: Pattern of care in adjuvant therapy for resected Stage I non-small cell lung cancer: real-world data from Japan. Jpn J Clin Oncol, 50:481, 2020
56. Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H. An Oncogenic Alteration Creates a Microenvironment that Promotes Tumor Progression by Conferring a Metabolic Advantage to Regulatory T Cells. Immunity, 53:187-203.e8, 2020
57. Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, Hara H, Baba H, Tsuda M, Hosaka H, Kawakami H, Oshima T, Omuro Y, Machida N, Esaki T, Yoshida K, Nishina T, Komatsu Y, Han SR, Shiratori S, Shitara K. Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: Cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer, 129:97-106, 2020
58. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol, 38:2053-2061, 2020
59. Doi T, Fujiwara Y, Koyama T, Ikeda M, Helwig C, Watanabe M, Vugmeyster Y, Kudo M. Phase I Study of the Bifunctional Fusion Protein Bintrafusp Alfa in Asian Patients with Advanced Solid Tumors, Including a Hepatocellular Carcinoma Safety-Assessment Cohort. Oncologist, 25:e1292-e1302, 2020
60. Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N, Higuchi T, Sato A, Kuwata T, Shitara K. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol, 21:1057-1065, 2020
61. Kubota Y, Kawazoe A, Sasaki A, Mishima S, Sawada K, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Ishii G, Kuwata T, Shitara K. The Impact of Molecular Subtype on Efficacy of Chemotherapy and Checkpoint Inhibition in Advanced Gastric Cancer. Clin Cancer Res, 26:3784-3790, 2020
62. Ishii T, Kawazoe A, Sasaki A, Mishima S, Kentaro S, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Kuwata T, Ishii G, Shitara K. Clinical and molecular factors for selection of nivolumab or irinotecan as third-line treatment for advanced gastric cancer. Ther Adv Med Oncol, 12:1758835920942377, 2020
63. Sasaki A, Kawazoe A, Eto T, Okunaka M, Mishima S, Sawada K, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Akimoto T, Shitara K. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer. ESMO Open, 4:2020
64. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, Yuki S, Wakabayashi M, Nomura S, Sato A, Kuwata T, Kawazu M, Mano H, Togashi Y, Nishikawa H, Yoshino T. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin Cancer Res, 26:5887-5894, 2020
65. Sasaki A, Nakamura Y, Togashi Y, Kuno H, Hojo H, Kageyama S, Nakamura N, Takashima K, Kadota T, Yoda Y, Mishima S, Sawada K, Kotani D, Kawazoe A, Kuboki Y, Taniguchi H, Kojima T, Doi T, Yoshino T, Yano T, Kobayashi T, Akimoto T, Nishikawa H, Shitara K. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer, 23:893-903, 2020
66. Mishima S, Kawazoe A, Shitara K. Safety of pembrolizumab in recurrent or advanced gastric cancer expressing PD-L1 refractory to platinum and fluoropyrimidine. Expert Opin Drug Saf, 19:1063-1068, 2020
67. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol, 6:1571-1580, 2020
68. Nakamura Y, Sasaki A, Yukami H, Jogo T, Kawazoe A, Kuboki Y, Taniguchi H, Yamashita R, Kuwata T, Ozawa M, Nakamura M, Yoshino T, Shitara K. Emergence of Concurrent Multiple EGFR Mutations and MET Amplification in a Patient With EGFR-Amplified Advanced Gastric Cancer Treated With Cetuximab. JCO Precis Oncol, 4:2020
69. Okunaka M, Kotani D, Demachi K, Kawazoe A, Yoshino T, Kawasaki T, Shitara K. Retrospective cohort study of nanoparticle albumin-bound paclitaxel plus ramucirumab versus paclitaxel plus ramucirumab as second-line treatment in patients with advanced gastric cancer. BMC Cancer, 20:1111, 2020
70. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, Rugo HS, de Braud F, Varga AI, Hansen A, Wang H, Krishnan S, Norwood KG, Doi T. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer, 147:2190-2198, 2020
71. Kondo S, Tajimi M, Funai T, Inoue K, Asou H, Ranka VK, Wacheck V, Doi T. Correction to: Phase 1 dose-escalation study of a novel oral PI3K/mTOR dual inhibitor, LY3023414, in patients with cancer. Invest New Drugs, 38:1846, 2020
72. Gelderblom H, Jones RL, George S, Valverde Morales C, Benson C, Jean-Yves Blay, Renouf DJ, Doi T, Le Cesne A, Leahy M, Hertle S, Aimone P, Brandt U, Sch?ffski P. Imatinib in combination with phosphoinositol kinase inhibitor buparlisib in patients with gastrointestinal stromal tumour who failed prior therapy with imatinib and sunitinib: a Phase 1b, multicentre study. Br J Cancer, 122:1158-1165, 2020
73. Tsurutani J, Iwata H, Krop I, J?nne PA, Doi T, Takahashi S, Park H, Redfern C, Tamura K, Wise-Draper TM, Saito K, Sugihara M, Singh J, Jikoh T, Gallant G, Li BT. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov, 10:688-701, 2020
74. Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, Bergsland E, Shah M, Fakih M, Takahashi S, Piha-Paul SA, O'Neil B, Thomas S, Lolkema MP, Chen M, Ibrahim N, Norwood K, Hadoux J. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin Cancer Res, 26:2124-2130, 2020
75. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol, 38:1887-1896, 2020
76. Nishida T, Doi T. A new approach to refractory gastrointestinal stromal tumours with diverse acquired mutations. Lancet Oncol, 21:864-865, 2020
77. Tabernero J, Alsina M, Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, Ghidini M, Faustino C, Gorbunova V, Zhavrid E, Nishikawa K, Ando T, Yal??n ?, Van Cutsem E, Sabater J, Skanji D, Leger C, Amellal N, Ilson DH. Health-related quality of life associated with trifluridine/tipiracil in heavily pretreated metastatic gastric cancer: results from TAGS. Gastric Cancer, 23:689-698, 2020
78. Tsumura R, Koga Y, Hamada A, Kuwata T, Sasaki H, Doi T, Aikawa K, Ohashi A, Katano I, Ikarashi Y, Ito M, Ochiai A. Report of the use of patient-derived xenograft models in the development of anticancer drugs in Japan. Cancer Sci, 111:3386-3394, 2020
79. Mehnert JM, Bergsland E, O'Neil BH, Santoro A, Schellens JHM, Cohen RB, Doi T, Ott PA, Pishvaian MJ, Puzanov I, Aung KL, Hsu C, Le Tourneau C, Hollebecque A, ?lez E, Tamura K, Gould M, Yang P, Stein K, Piha-Paul SA. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer, 126:3021-3030, 2020
80. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med, 383:1207-1217, 2020
81. Izawa N, Shitara K, Yonesaka K, Yamanaka T, Yoshino T, Sunakawa Y, Masuishi T, Denda T, Yamazaki K, Moriwaki T, Okuda H, Kondoh C, Nishina T, Makiyama A, Baba H, Yamaguchi H, Nakamura M, Hyodo I, Muro K, Nakajima TE. Early Tumor Shrinkage and Depth of Response in the Second-Line Treatment for KRAS exon2 Wild-Type Metastatic Colorectal Cancer: An Exploratory Analysis of the Randomized Phase 2 Trial Comparing Panitumumab and Bevacizumab in Combination with FOLFIRI (WJOG6210G). Target Oncol, 15:623-633, 2020
82. Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, Kinoshita T. Erratum: Author's Affiliation Correction. Type II human epidermal growth factor receptor heterogeneity is a poor prognosticator for type II human epidermal growth factor receptor positive gastric cancer (World J Clin Cases 2019; Aug 6; 7 (15): 1964-1977). World J Clin Cases, 8:5494-5495, 2020
83. Watanabe K, Mitsunaga S, Kojima M, Suzuki H, Irisawa A, Takahashi H, Sasaki M, Hashimoto Y, Imaoka H, Ohno I, Ikeda M, Akimoto T, Ochiai A. The "histological replacement growth pattern" represents aggressive invasive behavior in liver metastasis from pancreatic cancer. Cancer Med, 9:3130-3141, 2020
84. Kan M, Imaoka H, Watanabe K, Sasaki M, Takahashi H, Hashimoto Y, Ohno I, Mitsunaga S, Umemoto K, Kimura G, Suzuki Y, Eguchi H, Otsuru T, Goda K, Ikeda M. Chemotherapy-induced neutropenia as a prognostic factor in patients with pancreatic cancer treated with gemcitabine plus nab-paclitaxel: a retrospective cohort study. Cancer Chemother Pharmacol, 86:203-210, 2020
85. Yuda J, Odawara J, Minami M, Muta T, Kohno K, Tanimoto K, Eto T, Shima T, Kikushige Y, Kato K, Takenaka K, Iwasaki H, Minami Y, Ohkawa Y, Akashi K, Miyamoto T. Tyrosine kinase inhibitors induce alternative spliced BCR-ABL(Ins35bp) variant via inhibition of RNA polymerase II on genomic BCR-ABL. Cancer Sci, 111:2361-2373, 2020
86. Kuzume A, Chi S, Yamauchi N, Minami Y. Immune-Checkpoint Blockade Therapy in Lymphoma. Int J Mol Sci, 21:2020
87. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Reply to T.J.A. Dekker. J Clin Oncol, 38:3351-3352, 2020
88. Nagasaki J, Togashi Y, Sugawara T, Itami M, Yamauchi N, Yuda J, Sugano M, Ohara Y, Minami Y, Nakamae H, Hino M, Takeuchi M, Nishikawa H. The critical role of CD4+ T cells in PD-1 blockade against MHC-II-expressing tumors such as classic Hodgkin lymphoma. Blood Adv, 4:4069-4082, 2020
89. Shichijo T, Maruyama D, Yamauchi N, Maeshima AM, Sugano M, Yuda S, Tajima K, Kurihara H, Shimada K, Suzuki T, Toyoda K, Makita S, Fukuhara S, Munakata W, Suzuki T, Kobayashi Y, Taniguchi H, Minami Y, Izutsu K, Tobinai K. Transformation Scoring System (TSS): A new assessment index for clinical transformation of follicular lymphoma. Cancer Med, 9:8864-8874, 2020
90. Kogawa T, Fujii T, Wu J, Harano K, Fouad TM, Liu DD, Shen Y, Masuda H, Krishnamurthy S, Chavez-MacGregor M, Lim B, Murthy RK, Valero V, Tripathy D, Ueno NT. Prognostic Value of HER2 to CEP17 Ratio on Fluorescence In Situ Hybridization Ratio in Patients with Nonmetastatic HER2-Positive Inflammatory and Noninflammatory Breast Cancer Treated with Neoadjuvant Chemotherapy with or without Trastuzumab. Oncologist, 25:e909-e919, 2020
